Carbocations MCQ - Practice Questions with Answers
Quick Facts
-
Carbocations is considered one the most difficult concept.
-
17 Questions around this concept.
Solve by difficulty
The shape of carbocation is
The total number of optically active compounds formed in the following reaction is :

The order of stability of the following carbocations:
 is :
is :
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
What is the htbridisation state of benzyl carbonium ion

Arrange the following in increasing order of stability
(A)
(B)
(C)
(D)
(E)
Concepts Covered - 1
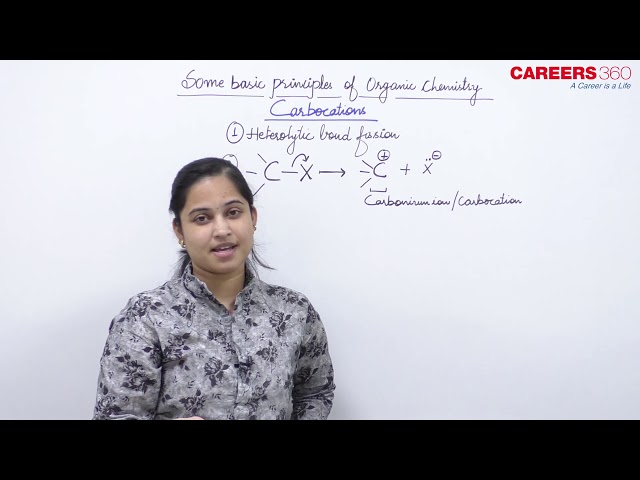
A species having a carbon atom possessing sextext of electrons and a positive charge is called a carbocation (earlier called carbonium ion). The +CH3 ion is known as a methyl cation or methyl carbonium ion. Carbocations are classified as primary, secondary or tertiary depending on whether one, two or three carbons are directly attached to the positively charged carbon. Carbocations are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects. The observed order of carbocation stability is: +CH3 < CH3C+H2 < (CH3)2C+H < (CH3)3C+. These carbocations have trigonal planar shape with positively charged carbon being sp2 hybridised. Thus, the shape of +CH3 maybe considered as being derived from the overlap of three equivalent C(sp2) hybridised orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as C(sp2)–H(1s) sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons. The shape of methyl carbocation is given as below:

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"