Organic Compounds - Classification Of Organic Compounds MCQ - Practice Questions with Answers
Quick Facts
-
13 Questions around this concept.
Solve by difficulty
How many $\pi$ bonds present in $CH_3CH=CHCN$?
The state of hybridization of ,
,
, and
of the hydrocarbon,
is in the following sequence:
Which one is the correct order of acidity?
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
The total number of $\pi$ - bond electrons in the following structure is:

The number of $\sigma$ bonds, $\pi$ bonds and lone pair of electrons in pyridine, respectively are:
Concepts Covered - 4
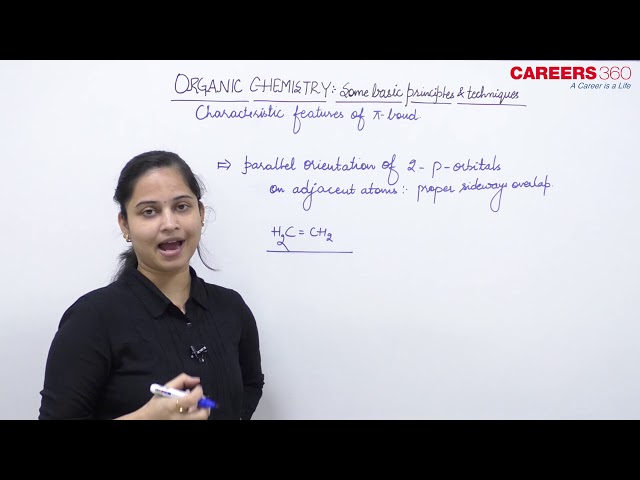
In a π (pi) bond formation, parallel orientation of the two p orbitals on adjacent atoms is necessary for a proper sideways overlap. Thus, in H2C=CH2 molecule all the atoms must be in the same plane. The p orbitals are mutually parallel and both the p orbitals are perpendicular to the plane of the molecule. Rotation of one CH2 fragment with respect to other interferes with maximum overlap of p orbitals and, therefore, such rotation about carbon-carbon double bond (C=C) is restricted. The electron charge cloud of the π bond is located above and below the plane of bonding atoms. This results in the electrons being easily available to the attacking reagents. In general, π bonds provide the most reactive centres in the molecules containing multiple bonds.
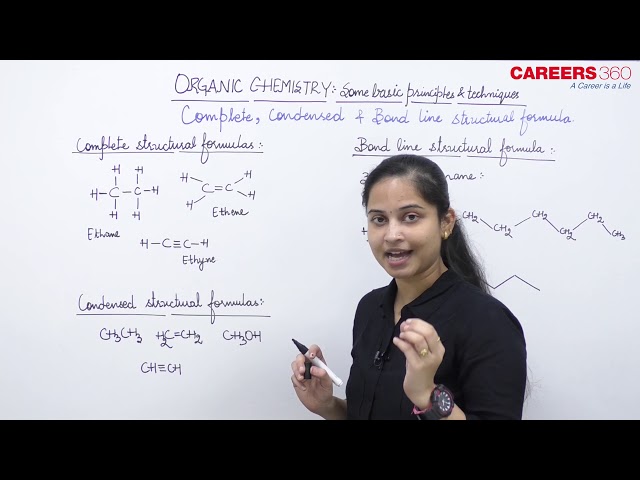
Complete structural formula
Such a structural formula focuses on the electrons involved in bond formation. A single dash represents a single bond, double dash is used for double bond and a triple dash represents triple bond. Lone-pairs of electrons on heteroatoms (e.g., oxygen, nitrogen, sulphur, halogens etc.) may or may not be shown. Thus, ethane (C2H6), ethene (C2H4), ethyne (C2H2) and methanol (CH3OH) can be represented by the structural formulas as shown below. Such structural representations are called complete structural formulas.


Condensed structural formula
These structural formulas can be further abbreviated by omitting some or all of the dashes representing covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. The resulting expression of the compound is called a condensed structural formula. Thus, ethane, ethene and ethyne can be written as:

Bond-line structural formula
In this bond-line structural representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The terminals denote methyl (–CH3) groups (unless indicated otherwise by a functional group), while the line junctions denote carbon atoms bonded to an appropriate number of hydrogens required to satisfy the valency of the carbon atoms. For example 3-Methyloctane is represented as follows:

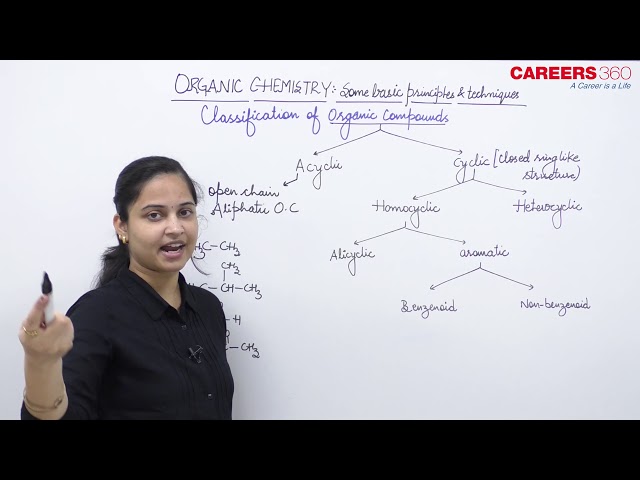
Acyclic or Open-chain compounds
These are the compounds in which the carbon atoms are linked to each other in such a manner that the molecule is having an open-chain structure. The chain of the carbon atoms may be straight or branched. These compounds are also called as aliphatic compounds. The term aliphatic has been derived from the Greek word aleiphatos meaning fats, since the earliest compounds to be studied were fatty acids or compounds found in fats. Some examples include:


Ethane Acetic acid
Cyclic or Closed-chain compounds
These are the compounds in which carbon atoms are linked to each other or to the atoms of other elements in such a manner that the molecule has a closed-chain or cyclic or ring structure. One or more close-chains or rings may be present in the molecule. The compounds with only one ring of atoms in the molecule are known as monocyclic but those with more than one ring of atoms are termed as polycyclic. These are divided into two categories:
(a) Homocyclic compounds: These are the compounds having a ring or rings of carbon atoms only in the molecule. The arbocyclic or hoomocyclic compounds may again be divided into two types, i.e,
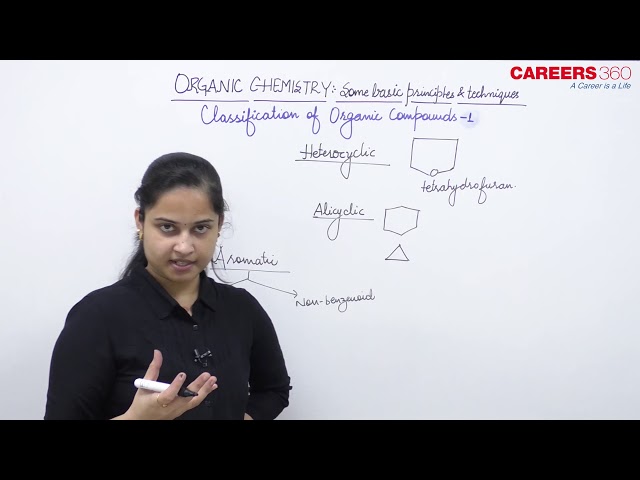
- Alicyclic compounds: These are the compounds which contain rings of three or more carbon atoms. These resemble with aliphatic compounds than aromatic compounds in many respects. That is why these are named alicyclic, i.e, aliphatic cyclic. Some examples include,

- Aromatic compounds: These compounds consist of at least one benzene ring, i.e, a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and hence, named as aromatic.

The above compounds are also known as benzenoid aromatics as their molecules consist of benzene ring or rings. However, there are aromatic compounds, which have structural units different from benzenoid type and are known as non-benzenoid aromatics.

(b) Heterocyclic compounds: These are cyclic compounds having ring or rings built up of more than one kind of atoms. The most common other atoms besides carbon are O, N and S. Some examples include,

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"