Displacement Reaction MCQ - Practice Questions with Answers
Quick Facts
-
Balancing of Redox Reaction: Ion Electrode Method, Balancing of Disproportionation Redox Reaction: Ion Electrode Method are considered the most difficult concepts.
-
Balancing of Redox Reaction: Oxidation Number Method are considered the most asked concepts.
-
37 Questions around this concept.
Solve by difficulty
As the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following?
Concepts Covered - 4
In a displacement reaction, an ion (or an atom) in a compound is replaced by an ion (or an atom) of another element. It may be denoted as:
Displacement reactions fit into two categories: metal displacement and non-metal displacement.
- Metal Displacement: A metal in a compound can be displaced by another metal in the uncombined state. Metal displacement reactions find many applications in metallurgical processes in which pure metals are obtained from their compounds in ores. A few such examples are:
In each case, the reducing metal is a better reducing agent than the one that is being reduced which evidently shows more capability to lose electrons as compared to the one that is reduced.
- Non-metal displacement: The non-metal displacement redox reactions include hydrogen displacement and a rarely occurring reaction involving oxygen displacement. All alkali metals and some alkaline earth metals (Ca, Sr, and Ba) which are very good reductants, will displace hydrogen from cold water.
Less active metals such as magnesium and iron react with steam to produce dihydrogen gas:
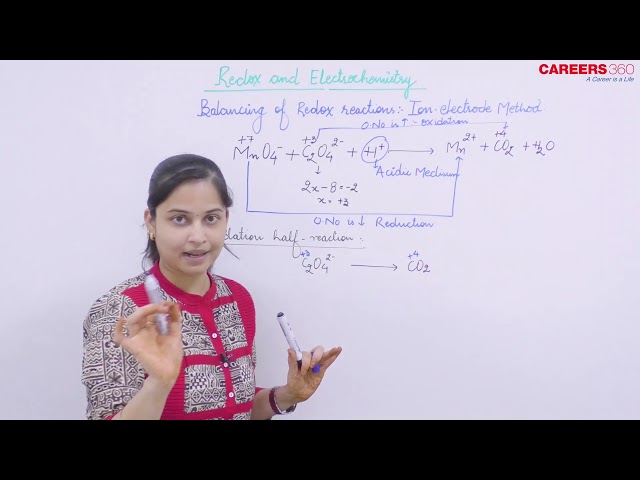
- Identify the oxidation and reduction half reactions and write them separately in ionic form.
For example:
Mn in MnO-4 in acidic medium generally goes to MnSO4 or Mn2+
Write equation like this:
- Balance each half reaction separately. This is done accordingly to the following procedure.
- Balance all the atoms of both reactions except 'O' and 'H'.
- Now balance O and H atoms depending upon the medium of reaction:
Acidic Medium
Now to balance H atoms, add as many H+ ions required to the side that is deficient in H atoms.
Basic Medium
To balance O atoms, add same number of H2O molecules to the side having excess of O atoms and add the double the number of OH- ions to the other side (i.e., to the side deficient in O atoms).
Now to balance H atoms, add same number of OH- ions to the side in excess of H atoms and then add same number of water molecules to the other side (i.e., the side deficient in H atoms).
Alternatively, for balancing in the basic medium, you can first balance in the acidic medium and then add as many OH- ions on both sides such that all the H+ on one side is consumed to give water. now complete the net number of water molecules after the above operation.
3. Now add electrons to the side deficient in negative charge in order to balance the charge on both sides.
These are balanced half reactions in acidic and basic medium respectively.
4. Now add two half reactions together in such a manner that electrons from both sides cancel. So multiply by coefficients so that number of electrons produced in oxidation equals the number of electrons used in reduction.
Disproportionation reactions are those reactions in which on species of some oxidation state converts into two different oxidation states, one oxidation state is higher and other is lower.
The balancing of disproportionation reaction by ion electrode method can be understood by the following example.
The chemical reaction is as follows:
In this reaction, Cl on reactant side has zero oxidation state but on product side its oxidation states are +5(in ClO3) and -1 in Cl-.
STEP 1: Write oxidation half-reaction
Now balance the chlorine atoms on both sides. Thus the balance equation is as follows:
Now chlorine atoms are changing its oxidation states from 0 to 5. Thus, there is a total exchange of 10 electrons. So, write the complete balanced equation as follows:
STEP 2: Write the reduction half-reaction
Now balance the chlorine atoms on both sides. Thus the balance equation is as follows:
Now in this equation, chlorine atoms are changing its oxidation states from 0 to -1. Thus, there is a total exchange of 2 electrons. So, write the complete balanced equation as follows:
Now balance the electrons exchange of equations (i) and (ii) and then add them both. Thus the final added equation is as follows:
STEP 3: Balance the charge
In equation(iii), there is a total of -12 charge on the product side and zero charge on the reactant side. Thus, to balance the charge on both sides, add the required number of OH- ions on the deficient side. Thus,
STEP 4: Balance the oxygen atoms
To balance the oxygen atoms, add the required number of H2O molecules on the deficient side.
This is the final balanced equation for the given disproportionation reaction by ion-electrode method.
While balancing a given reaction by this method, following steps are to be followed :
-
Assign oxidation state to each element (atom) on both sides of the equation and identify which element has been oxidised and which reduced.
-
Write two oxidation and reduction reactions (two half-reactions) separately involving only atoms. Now balance the atoms on both sides of equation in each half-reaction.
-
Balance charge on both sides by adding electrons to whichever side is deficient in electrons. (i.e., negative charge)
-
Add two half-reactions together. In doing this we want electrons to cancel from both sides. For this, multiply the equations by appropriate coefficients so that number of electrons produced in oxidation reaction equals to that used up in reduction reaction.
-
Now compare this balanced equation with original unbalanced equation. Here, notice whether the given equation is a molecular equation or an ionic equation.
-
For molecular equation, to balance Oxygen (O) and Hydrogen (H), add required water to the side deficient in H and check for Oxygen atoms on both sides. (They will be equal on both sides).
-
For ionic equation, apart from balancing O and H atoms, charge needs to be balanced. It depends upon the medium in which the reaction is taking place: Acidic (containing H+ ions or any acid) or Alkaline (containing OH- ions or any base).
-
In Acidic medium, count total charge on both sides and balance it by adding H+ ions to the required side (i.e., to the side deficient in +ve charge). Finally, add enough water molecules to balance H and O atoms to the required side.
-
In Basic medium, balance the charge by adding OH- ions to the side with excess of +ve charge and finally add required number of H2O molecules to the appropriate side to balance O and H.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"