Simultaneous And Series Disintegration MCQ - Practice Questions with Answers
Quick Facts
-
9 Questions around this concept.
Solve by difficulty
Directions: In this question, a word is represented by only one set of numbers as given in any one of the alternatives. The sets of numbers given in the alternatives are represented by two classes of alphabets as in the two matrices, given below. The columns and rows of Matrix (I) are numbered from 0 to 3 and those of Matrix (II) are numbered from 5 to 9. A letter from these matrices can be represented first by its row and next by its column, e.g. E can be represented by 00,11, etc. and I can be represented by 56, 86, etc. Similarly, you have to identify the set for the word 'TOLD'.
Concepts Covered - 1
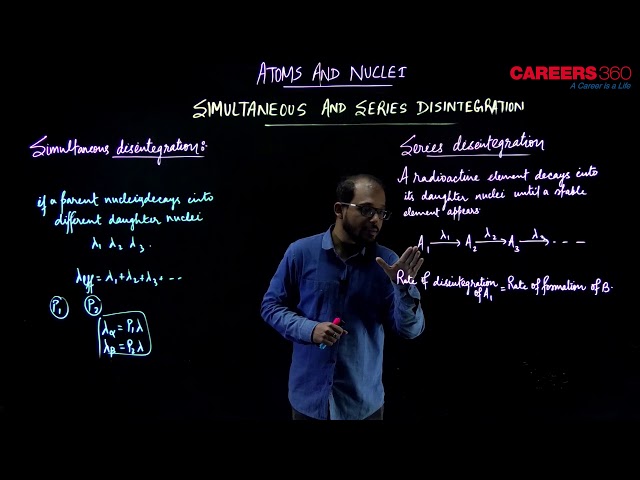
- Simultaneous decay-
As we know that due to radioactive disintegration a radio nuclide transforms into its daughter nucleus. Depending on the nuclear structure and its unstability, a parent nucleus may undergo either or
emission. Sometimes a parent nucleus may undergo both types of emission imultaneousonly.
If an element decays to different daughter nuclei with different decay constant etc. for each decay mode, then the effective decay constant of the parent nuclei can be given as
Similarly, for a radioactive element with decay constant which decays by both
and
decays given that the probability
for an -emission is
and that for
emission is
the decay constant of the element can be split for individual decay modes. Like in this case the decay constants for
and
decay separately can be given as
- Series decay-
Accumulation of Radioactive element in Radioactive series-
A radioactive element decays into its daughter nuclei until a stable element appears. Consider a radioactive series-
A radioactive element disintegrates to form another radioactive element
which in turn disintegrates to another element
and so on. Such decays are called Series or Successive disintegration.
Here, rate of disintegration of = Rate of formation of B
Therefore, net formation of B = Rate of disintegration of - Rate of disintegration of
=
If the Rate of disintegration of becomes equal to the Rate of disintegration of
, then it is called Radioactive equilibrium. So the equation become -
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"