Benzene Reactions - Sulfonation, Nitration and Halogenation MCQ - Practice Questions with Answers
Quick Facts
-
25 Questions around this concept.
Solve by difficulty
What products are formed when the following compound is treated with Br2 in the presence of FeBr3?

The reaction of toluene with Cl2 in presence of FeCl3 gives X and reaction in presence of light gives 'Y'. Thus, 'X' and 'Y' are
Phenol reacts with methyl chloroformate in the presence of NaOH to form product A. A reacts with Br2 to form product B. A and B are respectively :
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Nitrobenzene on reaction with conc. HNO3/H2SO4 at 80 - 100°C forms which one of the following products?
Nitrobenzene can be prepared from benzene by using a mixture of conc. HNO3 and conc. H2SO4 in the mixture, nitric acid acts as a/an:
Nitrobenzene on reaction with conc. HNO3/H2SO4 at 80 - 100°C forms which one of the following products ?
Concepts Covered - 2
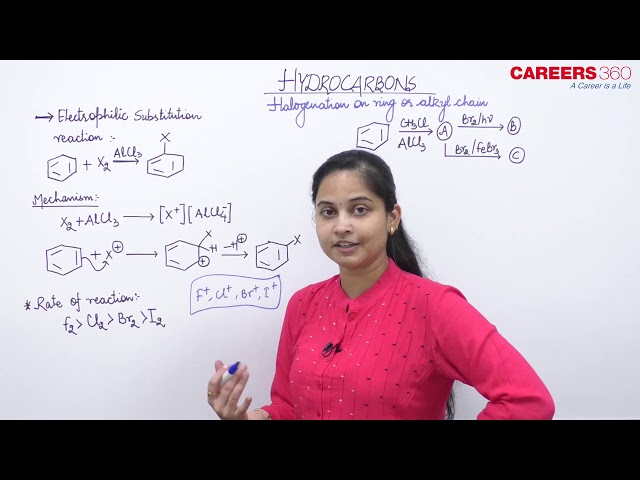
Benzene undergoes chlorination when it is treated with chlroine in presence of Lewis catalyst such as AlCl3 or Fe or FeCl3 and in absence of light.
For example:

Mechanism
The mechanism of this reaction follows two step:
NOTE: The hydrogen is removed by the AlCl−4 ion which was formed in the first stage. The aluminum chloride catalyst is re-generated in this second stage.
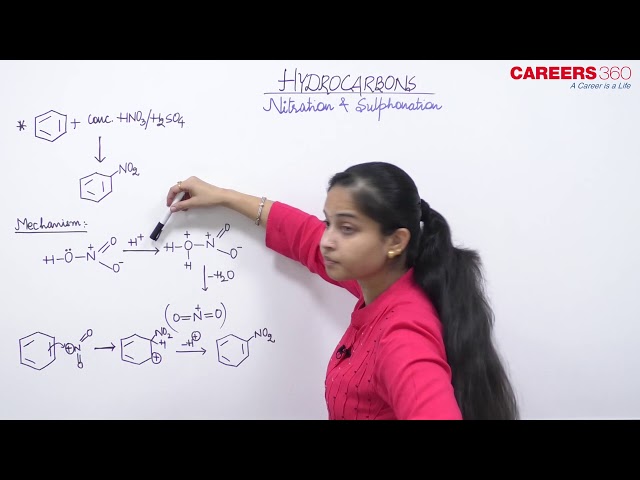
Nitration
Benzene undergoes nitration when treated with concentrated nitric acid inpresence off concentrated sulphuric acid, i.e, nitrobenzene is formed. The reaction is carried at 313-323K, when one of the H atom from the benene ring is replaced by nitro group.
For example:
Sulphonation
Benzene forms benzene sulphonic acid with hot concentrated sulphuric aicd while with fuming sulphuric acid or oleum at high temerature, m-benzene disulphonic acid is formed.
For example:
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"