Friedel-Crafts Reaction MCQ - Practice Questions with Answers
Quick Facts
-
25 Questions around this concept.
Solve by difficulty
Which of the following can be used as the halide component for Friedel Crafts reaction?
The metal that cannot be obtained by electrolysis of an aqueous solution of its salts is :
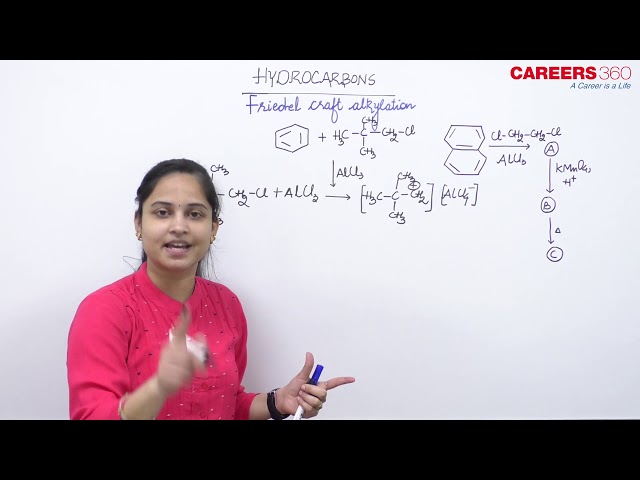
Benzene reacts with CH3Cl in the presence of anhydrous AlCl3 to form:
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
In which of the following reaction is a new C-C bond formed?
Which of the following compounds will not undergo Friedel - Craft's reaction easily:
Concepts Covered - 2
This reaction allowed for the formation of alkyl benzenes from alkyl halides. The reactivity of haloalkanes increases as we move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl than RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions.
For example:
Mechanism
- In this step a carbocation is formed that acts as the electrophile in the reaction. This step activates the haloalkane. Secondary and tertiary halides only form the free carbocation in this step.
- In this step, electrophilic attack on the benzene occurs that results in multiple resonance forms. The halogen reacts with the intermediate and picks up the hydrogen to eliminate the positive charge.
This electrophilic aromatic substitution allows the synthesis of monoacylated products from the reaction between arenes and acyl chlorides or anhydrides. The products are deactivated, and do not undergo a second substitution.
For example:

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"