Preparation of Alkynes MCQ - Practice Questions with Answers
Quick Facts
-
4 Questions around this concept.
Solve by difficulty
Which of the following reaction(s) can be used for the preparation of alkyl halides?
(I) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{HCl} \xrightarrow{\text { anh. } \mathrm{ZnCl}_2}$
(II) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{HCl} \rightarrow$
(III) $\left(\mathrm{CH}_3\right)_3 \mathrm{COH}+\mathrm{HCl} \rightarrow$
(IV) $\left(\mathrm{CH}_3\right)_2 \mathrm{CHOH}+\mathrm{HCl} \xrightarrow{\text { anh. } \mathrm{ZnCl}_2}$
Concepts Covered - 1
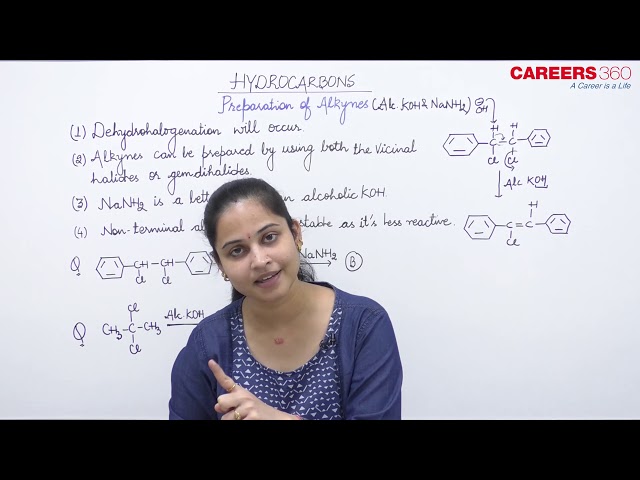
Calcium carbide: Ethyne is prepared by treating calcium carbide with water. Calcium carbide is prepared by heating quick lime with coke. Quick lime can be obtained by heating limestone as shown in the following reactions:
Vicinal dihalides: Vicinal dihalides on treatment with alcoholic potassium hydroxide undergo dehydrohalogenation. One molecule of hydrogen halide is eliminated to form alkenyl halide which on treatment with sodamide gives alkyne.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"