Alkyl Halides MCQ - Practice Questions with Answers
Quick Facts
-
Reaction with PCl5, PCl3, SOCl2 and HX is considered one the most difficult concept.
-
Nature of C-X bond and Physical Properties is considered one of the most asked concept.
-
42 Questions around this concept.
Solve by difficulty
The given compound

Is an example of ________
Which of the following reaction(s) can be used for the preparation of alkyl halides?
(I) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{HCl} \xrightarrow{\text { anh. } \mathrm{ZnCl}_2}$
(II) $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{HCl} \rightarrow$
(III) $\left(\mathrm{CH}_3\right)_3 \mathrm{COH}+\mathrm{HCl} \rightarrow$
(IV) $\left(\mathrm{CH}_3\right)_2 \mathrm{CHOH}+\mathrm{HCl} \xrightarrow{\text { anh. } \mathrm{ZnCl}_2}$
Strong reducing behaviour of $\mathrm{H}_3 \mathrm{PO}_2$ is due to:
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Concepts Covered - 5
Halogen atoms are more electronegative than carbon, therefore, carbon-halogen bond of alkyl halide is polarised; the carbon atom bears a partial positive charge whereas the halogen atom bears a partial negative charge. As we go down the group in the periodic table, the size of halogen atom increases. Fluorine atom is the smallest and iodine atom is the largest. Consequently, the carbon-halogen bond length also increases from to
.

The silver(I) salts of carboxylic acids react with halogens to give unstable intermediates which readily decarboxylate thermally to yield alkyl halides. The reaction is believed to involve homolysis of the C-C bond and a radical chain mechanism. In this reaction, ester is formed as a by-product. The reaction occurs as follows:
Mechanism
For example:

- Reaction with NaCN: NaCN or KCN is ionic in nature i.e, Na+CN-. Thus, the nucleophile in this case is CN-. The reaction occurs as follows:
For example:
- Reaction with NaNO2: NaNO2 or KNO2 is ionic in nature and it breaks into Na+ and NO-2. Nitrite ion NO-2 with structure O=N-O- is an ambient nucleophile with electron pairs on both N & O. NO-2 ion having an excess of electrons on O thus, allows it to act as a nucleophile in preference to N. The reaction occurs as follows:
For example:
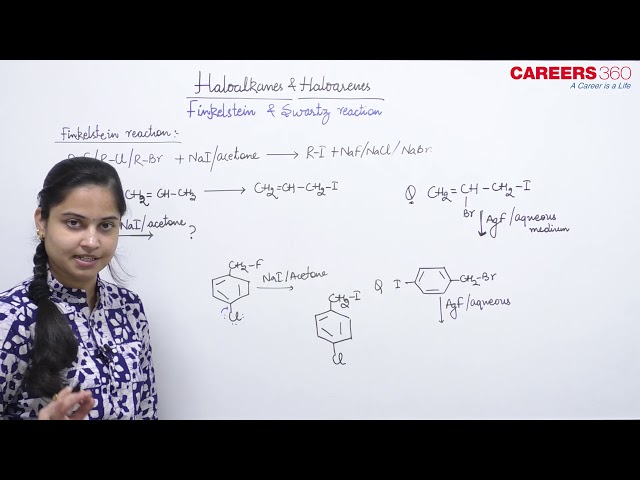
Finkelstein Reaction
Finkelstein's reaction is a method of preparation of alkyl iodides from alkyl chlorides or alkyl bromides. In this reaction, alkyl chlorides or bromides are treated with NaI in the presence of acetone to form alkyl iodides. The reaction occurs as follows:
We use NaI because it is soluble in acetone as it is covalent in nature. All other sodium halides are ionic in nature and thus not soluble.
For example:
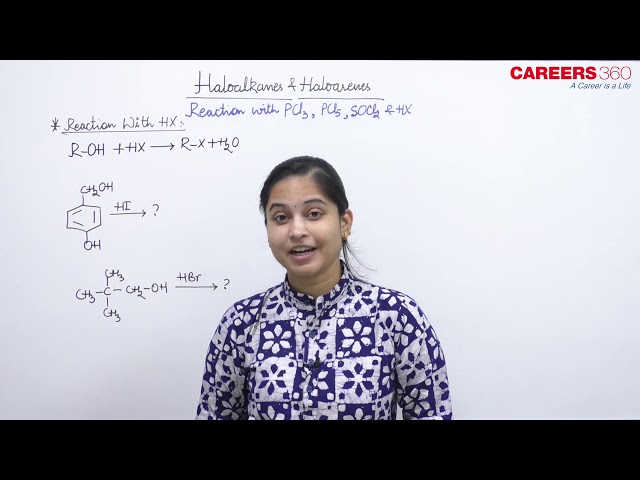
The reaction of alcohols ROH with PCl5 and PCl3 yields an alkyl halide RCl. The reactions of alcohols with PCl5, PCl3 and SOCl2 occurs as follows:
POCl3 and H3PO3 are generated in liquid phase and hence they are very hard to separate while SO2 and HCl are gases and thus they are easy to remove. Hence, for chlorination, we always use SOCl2 as the best option among the given reagents.
Mechanism
The reactions occurs as follows:
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"