Haloarene MCQ - Practice Questions with Answers
Quick Facts
-
13 Questions around this concept.
Solve by difficulty
The reaction of toluene with Cl2 in presence of FeCl3 gives 'X' and reaction in presence of light gives 'Y'. Thus, 'X' and 'Y' are:
Fluorobenzene $\left(C_6 H_5 F\right)$ can be synthesised in the laboratory
The reaction of $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}=\mathrm{CHCH}_3$ with HBr produces:
$p-H y d r o x y b e n z o p h e n o n e$ upon reaction with bromine in carbon tetrachloride gives:
Concepts Covered - 2
- From hydrocarbons by electrophilic substitution
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride.
-
The ortho and para isomers can be easily separated due to large difference in their melting points. Reactions with iodine are reversible in nature and require the presence of an oxidising agent (HNO3, HIO4) to oxidise the HI formed during iodination. Fluoro compounds are not prepared by this method due to high reactivity of fluorine.
-
From amines by Sandmeyer’s reaction
When a primary aromatic amine, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group by –Cl or –Br.
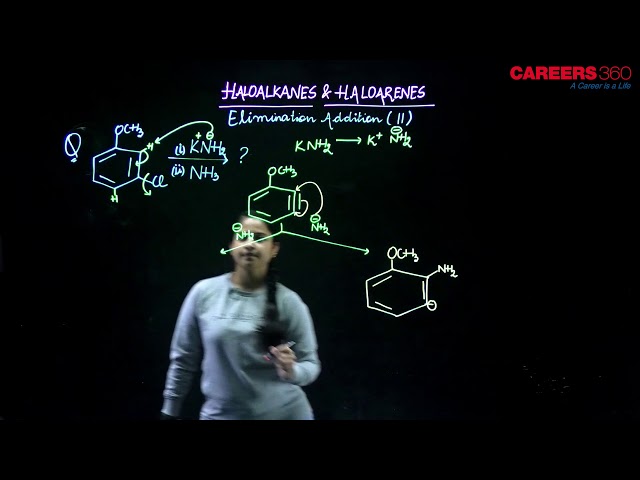
The accepted mechanism for nucleophilic aromatic substitution in nitro-substituted aryl halides is given as follows.
Mechanism
- Addition stage: The nucleophile, in this case methoxide ion, adds to the carbon atom that bears the leaving group to give a cyclohexadienyl anion intermediate.
- Elimination stage: Loss of halide form the cyclohexadienyl intermediate restores the aromaticity of the ring and gives the product of nucleophilic aromatic substitution.

Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"