Ionic Bond or Electrovalent Bond MCQ - NEET Practice Questions with Answers
Quick Facts
-
14 Questions around this concept.
Solve by difficulty
How does increasing ionic charge affect the melting and boiling points of a compound?
The enthalpy of solution for ionic compounds in water is:
Assertion: Ionic compounds are generally more stable than covalent compounds.
Reasoning: In ionic compounds, the strong electrostatic force of attraction between oppositely charged ions is responsible for the stability.
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Which of the following statements is true about an ionic bond?
Concepts Covered - 1
Ions are atoms or molecules bearing an electrical charge. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds or electrostatic forces of attraction between oppositely charged cations and anions. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they also have high melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are poor conductors of electricity as the strength of ionic bonds is very strong and it prevents the ions from moving freely in the solid state. Most ionic solids, however, dissolve readily in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because in the liquid state, these ions can move freely.
Neutral atoms and their associated ions have very different physical and chemical properties. For example, sodium atoms form sodium metal, a soft, silvery-white metal that burns vigorously in air and reacts explosively with water. Chlorine atoms form chlorine gas, Cl2, a yellow-green gas that is extremely corrosive to most metals and very poisonous to animals and plants. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt, which contains sodium cations and chloride anions. The compound composed of these ions exhibits properties entirely different from the properties of the elements sodium and chlorine. Chlorine is poisonous, but sodium chloride is essential to life; sodium atoms react vigorously with water, but sodium chloride simply dissolves in water.
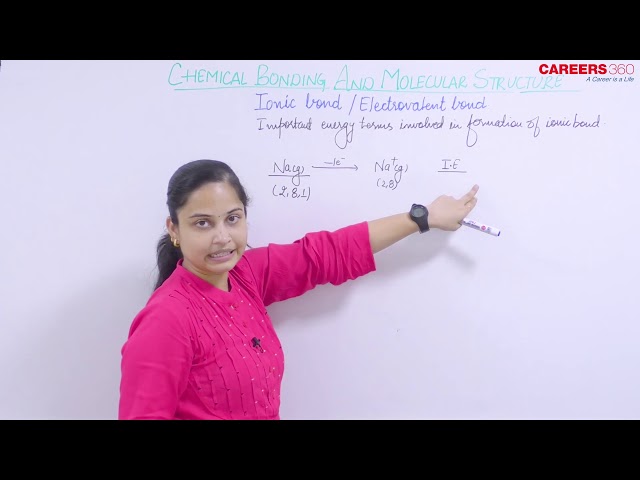
The Formation of Ionic Compounds
Binary ionic compounds are composed just of two elements i.e, a metal (which forms the cations) and a nonmetal (which forms the anions). For example, NaCl is a binary ionic compound. Many metallic elements have relatively low ionization potentials and lose electrons easily. These elements lie to the left in a period or near the bottom of a group on the periodic table. Nonmetal atoms have relatively high electron affinities and thus readily gain electrons lost by metal atoms, thereby filling their valence shells. Nonmetallic elements are found in the upper-right corner of the periodic table.
As all substances must be electrically neutral, the total number of positive charges on the cations of an ionic compound must be equal the total number of negative charges on its anions. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative charges.
It is important to consider that the formula for an ionic compound does not represent the physical arrangement of its ions. For example, sodium chloride (NaCl) “molecule”, because there is not a single ionic bond between any particular pair of sodium and chloride ions. The attractive forces between ions are isotropic i.e, the same in all directions in other words, any particular ion is equally attracted to all of the nearby ions of opposite charge. This results in the ions arranging themselves into a tightly bound, three-dimensional lattice structure. Sodium chloride, for example, consists of a regular arrangement of equal numbers of Na+ cations and Cl– anions as shown in the figure.

The strong electrostatic force of attraction between Na+ and Cl– ions hold them tightly together in solid NaCl. It requires 769 kJ of energy to dissociate one mole of solid NaCl into separate gaseous Na+ and Cl– ions:
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"