Formal Charge And Its Properties MCQ - Practice Questions with Answers
Quick Facts
-
8 Questions around this concept.
Solve by difficulty
In O3 molecule, the formal charge on the central oxygen atom is
Of the following sets which one does NOT contain isoelectronic species?
Concepts Covered - 1
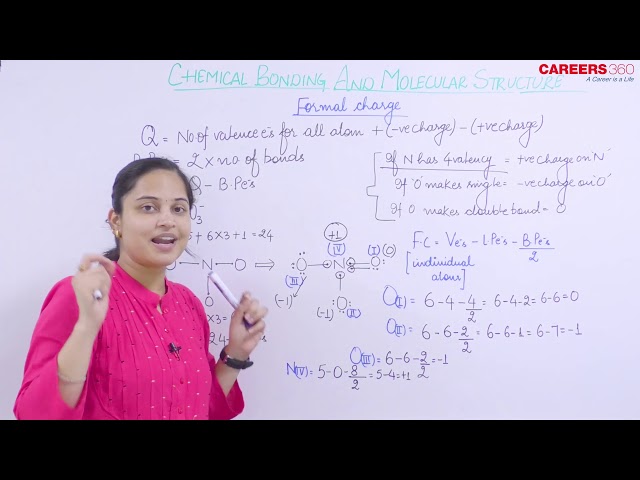
Formal Charge
The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. In other words, formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure.
Thus, we calculate the formal charge as follows:
formal charge = valence shell electrons − lone pair electrons − 1/2 bonding electrons
Using Formal Charge to Predict Molecular Structure
The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure—different multiple bonds and lone-pair electron placements or different arrangements of atoms, for instance. A few guidelines involving formal charge can be helpful in deciding which of the possible structures is most likely for a particular molecule or ion:
-
A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero.
-
If the Lewis structure must have nonzero formal charges, the arrangement with the smallest nonzero formal charges is preferable.
-
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign.
-
When we must choose among several Lewis structures with similar distributions of formal charges, the structure with the negative formal charges on the more electronegative atoms is preferable.
Let us consider some possible structures for carbon dioxide, CO2 and thiocyanate. We know that the less electronegative atom typically occupies the central position, but formal charges allow us to understand why this occurs. We can draw three possibilities for the structure: carbon in the center and double bonds, carbon in the center with a single and triple bond, and oxygen in the center with double bonds:

Comparing the three formal charges, we can identify the structure on the left as preferable because it has only formal charges of zero (Guideline 1).
In case of thiocyanate ion, an ion formed from a carbon atom, a nitrogen atom, and a sulfur atom, three different molecular structures: CNS–, NCS–, or CSN– are possible as shown below. The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown below:

Note that the sum of the formal charges in each case is equal to the charge of the ion (–1). However, the first arrangement of atoms is preferred because it has the lowest number of atoms with nonzero formal charges. Also, it places the least electronegative atom in the center and the negative charge on the more electronegative element.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"