Dragos Rule MCQ - Practice Questions with Answers
Quick Facts
-
15 Questions around this concept.
Solve by difficulty
$\mathrm{NH}_3$ is stronger lewis base in comparison to $\mathrm{PH}_3$ because:
Which of the following molecules has a bond angle of 120 degrees?
Which of the following molecules has the largest bond angle?
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Concepts Covered - 1
Bond Angle
Bond angle is the angle between two bonds that formed between two atoms. The figure given below illustrates the concept.

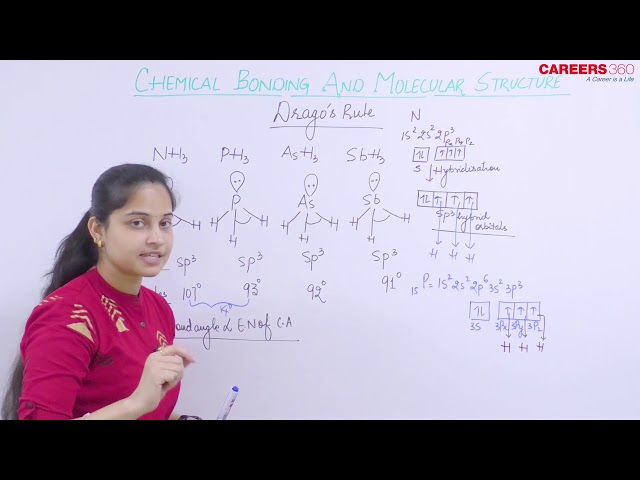
Drago’s Rule
Drago’s rule is an empirical rule that is used to explain the bond angles of hydrides of group 14, 15 and 16 and 2nd member of each of these three groups.
According to Drago’s rule when the various conditions are satisfied as mentioned below, then the energy difference will be very high between the participating atomic orbitals and hence no mixing of orbitals or hybridization takes place.
-
At least one lone pair must be present on the central atom.
-
The central atom must be of or below 3rd period.
-
The electronegativity of the surrounding atoms must be less than or equal to 2.1.
-
For these hydrides, hybridization does not take place and thus bonding takes place only through pure atomic p orbitals in PH3 and hence the bond angle will be approximately 900.
For example, the bond angle for H2O is 104.50 but for S2H, Se2H and Te2H, the bond angles are approximately 900.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"