Real Gas And Equation MCQ - Practice Questions with Answers
Quick Facts
-
6 Questions around this concept.
Solve by difficulty
A real gas behaves like an ideal gas if
The number of molecules in of water is closed to -
Find the dimension of the constant a in the van der Waals equation
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
In the following question, a statement of assertion (A) is followed by a statement of reason (R)
(A) Both the assertion and the inference are correct, and the inference is the correct interpretation of the assertion.
(B) Both the assertion and the inference are correct, but the inference is not a correct interpretation of the assertion.
(C) The statement is true but the reason is false.
(D) The assertion is false, but the reasoning is correct.
Assertion: The compressibility factors of H2 and He are $\left(1+\frac{Pb}{RT}\right)$
Reason: The compressibility factors of H2 and H can be derived from the van der Waals equation.
Concepts Covered - 1
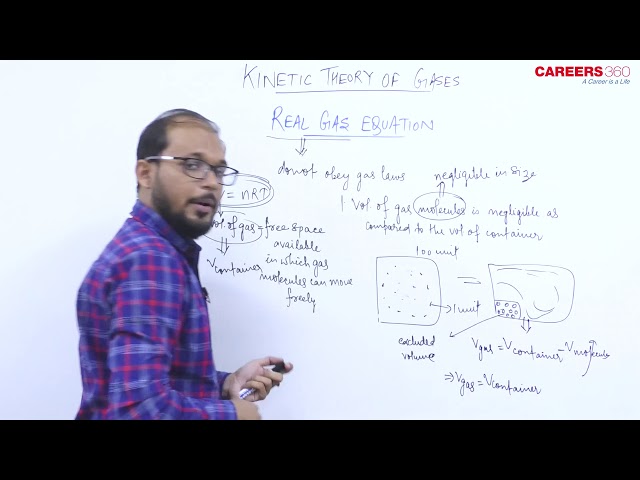
- Real gas- The gases which do not obey gas Laws are called Real gas.
Two main factors because of which Real gas deviates from ideal gas are:
1) Presence of force of attraction between molecules.
2) The size of molecules is not negligible.
The gases actually found in nature are called real gases.
From the ideal gas equation, we get
For exactly one mole of an ideal gas $\frac{P V}{R T}=1$
The quantity $\frac{P V}{R T}$ is called the compressibility factor and should be a unit for an ideal gas.
Plotting the experimentally determined value of $\frac{P V}{R T}$ for exactly one mole of various real gases as a function of pressure $P$
shows a deviation from identity as shown in the below graph.

Similarly, real gases show deviation from ideal behavior as a function of temperature as shown in the below graph.

From the above graphs, we can say that A real gas behaves as an ideal gas most closely at low pressure and high temperature.
- Real gas equation- The real gas equation, For n moles of gas, is given by
$$
\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T
$$
Where $a$ and $b$ are called Vander wall's constant having dimensions and units as follows:
Dimension : $[a]=\left[M L^5 T^{-2}\right]$ and $[b]=[L]$
Units : $a=N \times m$ and $b=m$
As we know the ideal gas equation is PV=nRT...... (2)
From equation (1) and (2) we can say that
The real gas equation is nothing but the ideal gas equation with two corrections (i.e Volume correction and Pressure correction)
These corrections are given by Vander Waal. So the real gas equation is also known as Vander Waal's gas equation.
1. Volume correction- Due to the finite size of the molecule the effective volume of gas becomes (V –nb).
2. Pressure correction- Due to the presence of intermolecular force in real gases, the effective pressure of gas becomes $P+\frac{n^2 a}{V^2}$
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"