Reversible And Irreversible Process MCQ - Practice Questions with Answers
Quick Facts
-
3 Questions around this concept.
Solve by difficulty
Which statement is true for a reversible thermodynamic process.
Which of the following conditions is true for a process to be reversible
Which of the following is an example of irreversible process
Concepts Covered - 1
- Reversible Process-
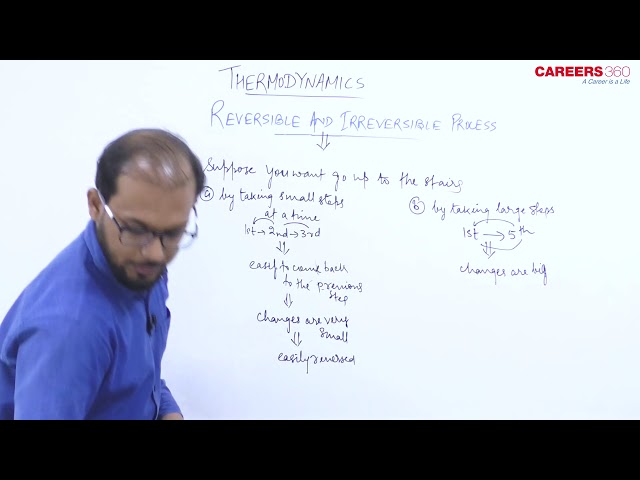
A reversible process is one that can be reversed in such a way that all changes occurring in the direct process are exactly repeated in the opposite order and inverse sense.
And in the Reversible Process, no change is left in any of the bodies taking part in the process, or in the surroundings.
Or " A process is reversible only if it is quasi-static and there is no dissipative effect."
Condition of a reversible process
1) The complete absence of dissipative force.
2) The process should be infinitely slow.
3) The temperature of the system must not differ appreciably from the surroundings.
Examples of the reversible process -
A reversible process is only an ideal concept. In the actual process, there is always a loss of heat due to friction,
conduction, radiation, etc. I.e No process is reversible in the true sense.
Some examples of reversible processes are:
- All isothermal and adiabatic changes are reversible if they are performed very slowly.
- Very slow evaporation or condensation.
- An extremely slow extension or contraction of spring without setting up oscillations.
- Irreversible process-
Any process which is not reversible exactly is an irreversible process. All-natural processes such as conduction, radiation, radioactive decay, etc. are irreversible.
Some examples of irreversible processes are :
- Sudden expansion or contraction
- Rapid evaporation or condensation
- The sudden and fast stretching of a spring
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"