Thermodynamic Equilibrium MCQ - Practice Questions with Answers
Quick Facts
-
7 Questions around this concept.
Solve by difficulty
A system can said to be in thermodynamic equilibrium if
"If the temperature of working substance must not differ appreciably from that of the surrounding of any stage of the cycle of the operations" is the condition of
In the given diagram if Body A and Body B are separately in thermal equilibrium with C Then-

Concepts Covered - 1
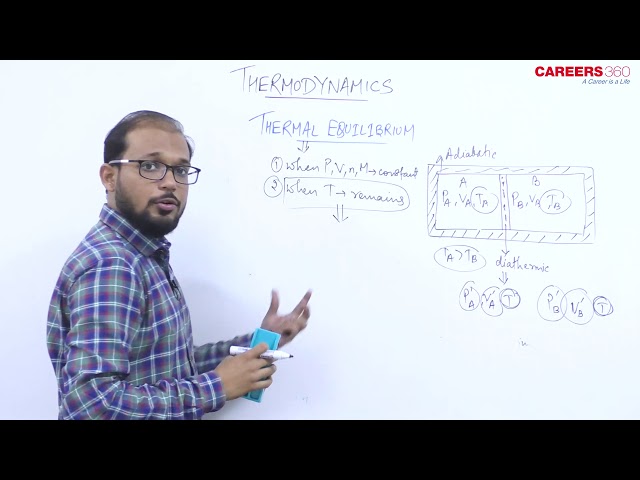
Thermodynamic equilibrium : When all the thermodynamic variables attain a steady value i.e. they do not change with respect to time, the system is said to be in the state of thermodynamic equilibrium. For the system to attain thermodynamic equilibrium, the following equilibrium must be attained -
(i) Mechanical equilibrium : There is no unbalanced force between the system and its surroundings. There is no pressure gradient.
(ii) Thermal equilibrium : There is a uniform temperature in all parts of the system and is same as that of surrounding. There is no temperature gradient.
(iii) Chemical equilibrium : There is a uniform chemical composition throughout the system and the surrounding. There is no concentration gradient.
Quasi-static process - A quasi-static process is a thermodynamic process which happens slowly enough for the system such that each state will remain in internal equilibrium.
Example of quasi-static compression - when the volume of a system changes at enough slow rate to allow the pressure to remain constant throughout the system
Zeroth Law of Thermodynamics.
If systems A and B are each in thermal equilibrium and B and C are in thermal equilibrium with each other, then A and C are in thermal equilibrium with each other.

Zeroth law leads to the concept of temperature. All bodies in thermal equilibrium must have a common property. This common property is called temperature.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"