Isochoric Process MCQ - Practice Questions with Answers
Quick Facts
-
12 Questions around this concept.
Solve by difficulty
In an isochoric process, if . Then
will be equal to
In thermodynamic processes, which of the following statements is not true?
Concepts Covered - 1
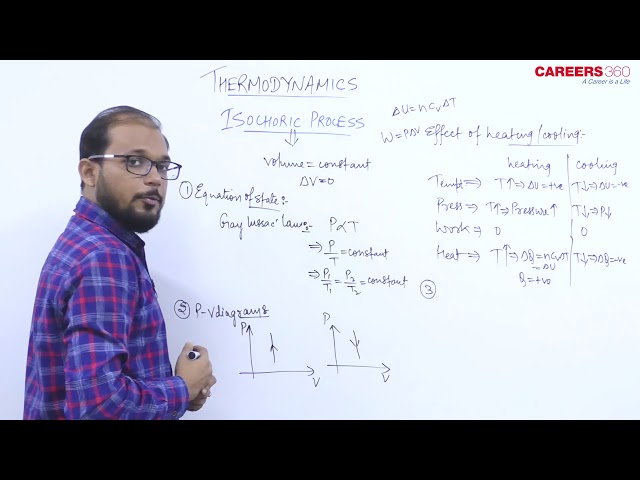
Isochoric Process- A Thermodynamic process in which volume remains constant is known as the Isochoric Process.
In this process, P and T change keeping P constant. So Gay-Lussac’s law is obeyed in this process.
Key points in the Isochoric Process
- Its Equation of state is given as $\frac{P}{T}=$ constant
$$
\text { or } \frac{P_1}{T_1}=\frac{P_2}{T_2}=\text { constant }
$$
P-V Indicator diagram for an isobaric process
Its PV graph has slope= infinity (i.e $\frac{d P}{d V}=\infty$,
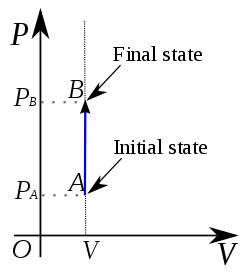
The above Graph represents an Isochoric increase in pressure at volume V.
The P-V diagram for this process is a line is parallel to the pressure line.
- Specific heat of gas during the Isochoric process is given by
$$
C_V=\frac{f}{2} R
$$
- The bulk modulus of elasticity during the Isochoric process is given by
$$
K=\frac{\Delta P}{-\Delta V / V}=\frac{\Delta P}{0}=\infty
$$
- Work done in the Isochoric process-
$$
\begin{aligned}
& \Delta W=P \Delta V \\
& \text { and as } \Delta V=0 \\
& \text { So } \Delta W=0
\end{aligned}
$$
- Internal energy in the Isochoric process
$$
\Delta U=n C_V \Delta T=n \frac{R}{(\gamma-1)} \Delta T
$$
- Heat in the Isochoric process
From FLTD $\Delta Q=\Delta U+\Delta W$
But $\Delta W=0$
$$
\Delta Q=\Delta U=n C_V \Delta T=n \frac{R}{(\gamma-1)} \Delta T=\frac{P_f V_f-P_i V_i}{\gamma-1}
$$
-
Examples of Isochoric process-
-
Heating of water in a pressure cooker (Valve closed)
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"