Freundlich Isotherm MCQ - Practice Questions with Answers
Quick Facts
-
Freundlich Isotherm is considered one the most difficult concept.
-
21 Questions around this concept.
Solve by difficulty
According to Freundlich adsorption isotherm, which of the following is correct ?
For a linear plot of log(x/m) versus log(p) in a Freundlich adsorption isotherm, which of the following statements is correct? (k and n are constants)
In Freundlich Adsorption isotherm, the value of 1/n is :
Concepts Covered - 0
Freundlich adsorption isotherm:
Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation:
(n>1)
where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P, k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
The relationship is generally represented in the form of a curve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure (Fig). These curves indicate that at a fixed pressure, there is a decrease in physical adsorption with increase in temperature. These curves always seem to approach saturation at high pressure.

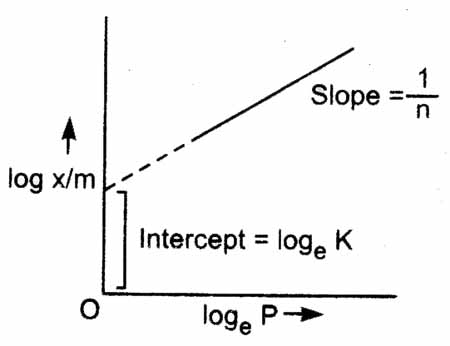
Logarithmic graph of Freundlich isotherm
Taking logarithm of eq. we get
The validity of Freundlich isotherm can be verified by plotting log x m on y-axis (ordinate) and log p on x-axis (abscissa). If it comes to be a straight line, the Freundlich isotherm is valid, otherwise not (Fig.). The slope of the straight line gives the value of . The intercept on the y-axis gives the value of log k.
"Stay in the loop. Receive exam news, study resources, and expert advice!"
