Second Order Reaction MCQ - Practice Questions with Answers
Quick Facts
-
11 Questions around this concept.
Solve by difficulty
The half-life of a second-order reaction is dependent on concentration of Reactant:-
For a second-order reaction, if the concentration of the reactant is tripled, the rate of the reaction becomes:.
A reaction follows second-order kinetics with the rate concentration of the reactant is 0.1 mott', what will be its concentration of ter 100 seconds?
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Concepts Covered - 1
Consider the reaction
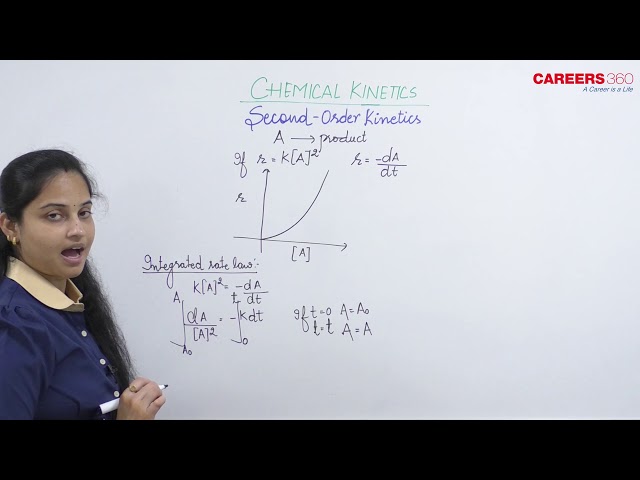
For ssecond order reaction, the rate law is given as follows:
- Integrated Rate Law: Since it is a second order reaction, thus:
Integrating both sides, we get:
where, Ao is the initial concentration of A at time t = 0
A is the remaining concentration of A after time 't'
- Half-life(t1/2): We know that the half-life for a reaction is the time when the concentration of the reactant(A) is half of tis initial value.
Thus, at time t = t1/2, A = Ao/2
From equation (i) we have:
This is the half-life for the second-order reaction.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"