Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Methods of Preparation of Carboxylic Acids, Acidity in Carboxylic Acids, Benzoin Condensation, Benzil-Benzilic Acid Rearrangement is considered one of the most asked concept.
82 Questions around this concept.
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their:
Which of the following statements about the depletion of the ozone layer is correct?
Major product of the following reaction is :

NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
$\mathrm{CH}_3-\mathrm{CH}(\mathrm{OH}) \mathrm{CH}_3+\mathrm{Br}_2 \xrightarrow{\mathrm{Na}_2 \mathrm{CO}_3 / \Delta} \mathrm{CH}_3-\mathrm{CO} \overline{\mathrm{O}} \mathrm{Na}^{+}+(\mathrm{P})$
product (P) is
The aldehydes which will NOT form Grignard product with one equivalent Grignard reagents are :
In the following sequence of reactions $\mathrm{CH}_3-\mathrm{Br} \xrightarrow{\mathrm{KCN}} \mathrm{A} \xrightarrow{\mathrm{H}_3 \mathrm{O}^{+}} \mathrm{B} \xrightarrow[\text { ether }]{\mathrm{LiAlH}_4} \mathrm{C}$, the end product (C) is:
Which dicarboxylic acid in presence of a dehydrating agent is least reactive to give an anhydride ?
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
In a set of reactions, ethyl benzene yielded a product D.

'D' would be :
Consider the following equilibrium
$\mathrm{AgCl} \downarrow+2 \mathrm{NH}_3 \rightleftharpoons\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right]^{+}+\mathrm{Cl}^{-}$
White precipitate of AgCl appears on adding which of the following ?
Consider the coordination compound, [Co(NH3)6]Cl3.
In the formation of this complex, the species which acts as the Lewis acid is :
From Esters
Esters on acidic hydrolysis give acids, while on basic hydrolysis give carboxylates, which on acidification give corresponding acids. The reaction occurs as follows:

From Grignard reagents
Grignard reagents with dry ice forms salts of carboxylic acids which after acidification give corresponding carboxylic acids. The reaction occurs as follows:

From Nitriles and Amides
Nitriles are hydrolysed in acidic or basic medium first to amides and then to acids. The reaction occurs as follows:

Formation of Anhydride
Carboxylic acid on heating with H2SO4 or P2O5 gives corresponding anhydride. The reaction occurs as follows:

Reaction with Ammonia
On heating with NH3, carboxylic acid gives ammonium salt which on further heating at high temperature gives amides. The reaction occurs as follows:

Reduction
Carboxylic acids are reduced to 1o alcohol by LiALH4 or better with B2H6. B2H6 does not easily reduce esters, nitro, and halo groups, and NaBH4 does not reduce (-COOH) group. The reaction occurs as follows:

Decarboxylation
When sodium salts of carboxylic acids are heated with soda lime (NaOH and CaO in 3:1 ratio), they form hydrocarbons by losing CO2. This is known as decarboxylation. The reaction occurs as follows:

Kolbe's electrolysis
The electrolysis of aqueous solution of sodium or potassium salts of carboxylic acid makes the carboxylic acid to undergo decarboxylation to form alkane. The reaction occurs as follows:

Halogenation
The method of preparing α-chloro or α-bromo acid is by Hell-Volhard-Zelinsky reaction, which is carried out by treating the acid with Cl2 or Br2 in the presence of a small amount of red phosphorous. The reaction occurs as follows:

Ring substitution
Aromatic carboxylic acid undergoes substitution electrophilic reactions in which the (-COOH) group acts as a deactivating and meta-directing group. It does not undergo Friedel-Crafts reaction because the (-COOH) group is deactivating and the catalyst AlCl3 gets bonded to the (COOH) group. The reactions occur as follows:


Carboxylic acids are weaker than mineral acids but they are stronger than alcohols, phenols and peroxy acids.
Aromatic aldehydes when heated with the anhydride of an aliphatic acid (containing two -H atoms) in the presence of its sodium or potassium salt result in condensation to form α,β-unsaturated acid.
Mechanism

For example,

Ketones and aldehydes react with α-bromoesters(BrCHRCOOEt) and Zn in benzene to form β-hydroxy esters. First the zinc organometallic BrZnCHRCOOEt is formed and then it adds to the (C=O).
Mechanism

For example,

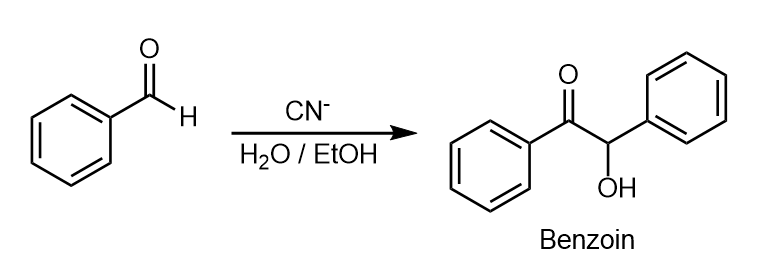
Benzoin condensation
When benzaldehyde is refluxed with aq. alcoholic KCN solution to give benzoin(-hydroxy ketone), the process is called benzoin condensation.
Mechanism

For example,
Benzil-Benzilic acid rearrangement
-Diketones undergo a rearrangement when treated with base (NaOH) to give
-hydroxy acids.
Mechanism

For example,

"Stay in the loop. Receive exam news, study resources, and expert advice!"
