Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Hydration, Hydroboration and Oxidation of Alkynes is considered one the most difficult concept.
Hydrohalogenation and Halogenation of Alkynes is considered one of the most asked concept.
15 Questions around this concept.
Which of these will not react with acetylene?
In the reaction

X and Y are :
Consider the following reactions:
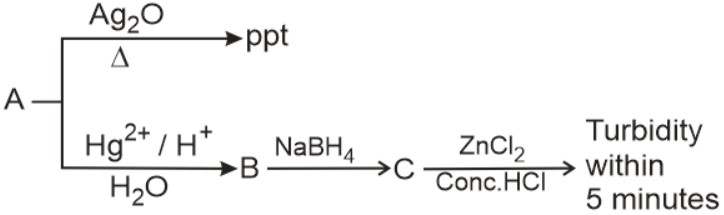
'A' is :
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Predict the correct intermediate and product in the following reaction.
An alkyne (P) on hydration with $\mathrm{H}_2 \mathrm{SO}_4$ in the presence of $\mathrm{HgSO}_4$ forms 3 - Methylbutan - 2 one as the major product. The alkyne $(\mathrm{P})$ is
In the reaction given

The major product (P) obtained is
Consider the reaction sequence given below,
The molar mass of the product(P) in the above reaction sequence is
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
The increasing order of the boiling points for the following compounds is :

Whar is the product formed during the reaction given below:
$\mathrm{CH} \equiv \mathrm{CH}+\underset{(2 \mathrm{eq.})}{\mathrm{HOCl}} \xrightarrow{0^{\circ} \mathrm{C}}$
On heating C2H2 to red heat, the compound formed is
Hydrohalogenation
Addition of one molecule of halogen gives vinyl halide which then adds another molecule of hydrogen halide to form gem-dihalide. This addition follows Markownikoff's rule. The reaction occurs as follows:

For example:

Halogenation
Alkenes combine with gaseous chlorine or bromine in the dark to form di or tetrahalides. Here the addition is Anti Markownikoff's. The reaction occurs as follows:

For example:

Hydration
Alkynes cannot be hydrated more easily than alkenes because of their low reactivity towards electrophilic addition reactions. The reaction occurs as follows:

Mechanism

For example:

Hydroboration-Oxidation reaction
Alkynes react with BH3 (in THF) and finally converted into carbonyl compounds. This method is useful for preparing aldehyde from terminal alkyne, which is otherwise not possible by hydration. The reaction occurs as follows:
For example:
Reaction with carbonyls
This reaction is very useful for the preparation of alcohols. In this reaction, a salt like NaNH2 is used which produces a carbanion as follows:
Now this carbanion reacts with the carbonyl group. Here, the carbanion binds with the carbonyl carbon and carbon-oxygen bond shifts to the oxygen giving it a negative charge. Now we use H3O+ to bind with O- and thus forms the OH or alcohol.
The complete mechanism is given below:
Addition of Hypochlorous acid
Alkynes when passed into hypochlorous acid solution forms dichloroacetaldehyde. The reaction occurs as follows:


For example:

Polymerisation
Alkynes polymerize to give the following compounds. The reactions occur as follows:

"Stay in the loop. Receive exam news, study resources, and expert advice!"
