Apply to Amrita Vishwa Vidyapeetham Allied & Life Science 2024
First Order Reaction - Practice Questions & MCQ
Quick Facts
-
First Order Reaction, Half Life of First Order Reaction, Graphs of First Order Kinetics are considered the most difficult concepts.
-
20 Questions around this concept.
Solve by difficulty
can be taken as the time taken for the concentration of a reactant to drop to 3/4 of its initial value. If the rate constant for a first order reaction is
the
can be written as
The rate equation for the reaction is found to be : rate
The correct statement in relation to this reaction is that the
The half-life period of a first-order reaction is 15 minutes. The amount of substance left after one hour will be :
Units of the rate constant of first and zero-order reactions in terms of molarity M unit are respectively.
Half life period of a first-order reaction is 1386 seconds. The specific rate constant of the reaction is:
For the first order reaction products, the plot of
vs time ,where
and
refer to concentartion at time
and
respectively ,is
Concepts Covered - 4
The rate of the reaction is proportional to the first power.


The chemical reaction occurs as follows:
R P
a 0
a-x x
We have,
[differentiate rate law]
Unit of
We know that the first-order equation is given as follows:
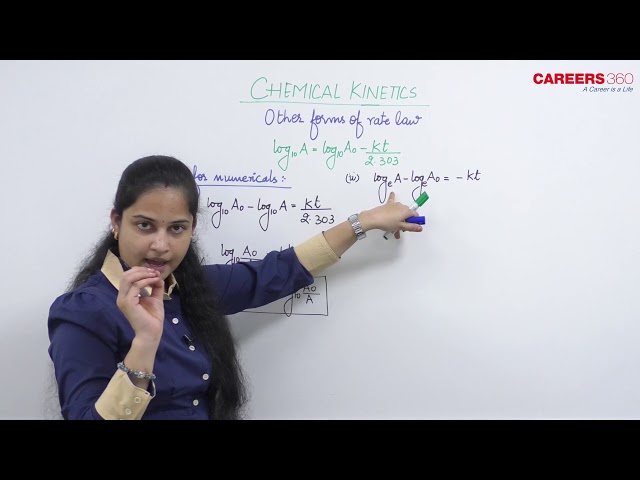
But there are other forms of rate law also available that we use for different purposes. These forms are mentioned below:
- Use to solve numericals:
- Exponential form:
This equation is also known as exponential form.
The half-life of a reaction is the time in which the concentration of a reactant is reduced to one half of its initial concentration. It is represented as t1/2.
For a zero order reaction, rate constant is given as:
The rate constant at t1/2 becomes:
It is clear that t1/2 for a zero order reaction is directly proportional to the initial concentration of the reactants and inversely proportional to the rate constant.
For the first order reaction,
So, the above equation becomes





Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"