Group 16 Elements (Oxygen Family) MCQ - NEET Practice Questions with Answers
Quick Facts
-
Group 16: Oxyen Family - Physical Properties, Chemical Properties - 1, Chemical Properties - 2 is considered one of the most asked concept.
-
51 Questions around this concept.
Solve by difficulty
Identify the wrong statement in the following
In which of the following arrangements the given sequence is not strictly according to the property indicated against it?
Concepts Covered - 3
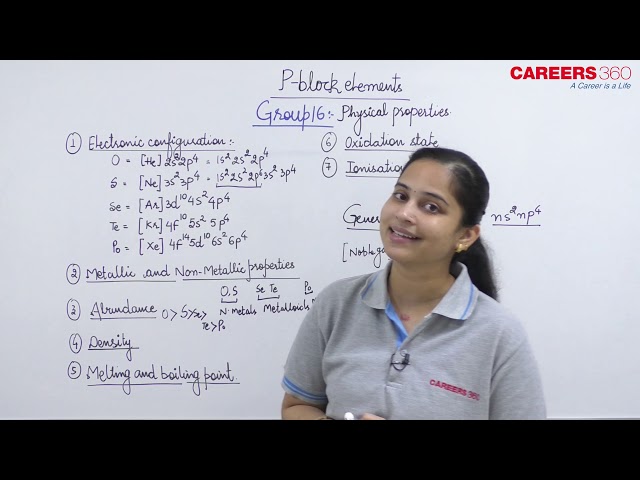
Some of the physical properties of Group 16 elements are given in Table 7.6. Oxygen and sulphur are non-metals, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived (Half-life 13.8 days). All these elements exhibit allotropy. The melting and boiling points increase with an increase in atomic number down the group. The large difference between the melting and boiling points of oxygen and sulphur may be explained on the basis of their atomicity; oxygen exists as diatomic molecule (O2) whereas sulphur exists as polyatomic molecule (S8).
Oxidation states and trends in chemical reactivity
The elements of Group 16 exhibit a number of oxidation states (Table 7.6). The stability of -2 oxidation state decreases down the group. Polonium hardly shows –2 oxidation state. Since electronegativity of oxygen is very high, it shows only negative oxidation state as –2 except in the case of OF2 where its oxidation state is +2. Other elements of the group exhibit +2, +4, +6 oxidation states but +4 and +6 are more common. Sulphur, selenium and tellurium usually show + 4 oxidation state in their compounds with oxygen and +6 with fluorine. The stability of +6 oxidation state decreases down the group and stability of +4 oxidation state increases (inert pair effect). Bonding in +4 and +6 oxidation states is primarily covalent.
- Reactivity with hydrogen: All the elements of Group 16 form hydrides of the type H2E (E=O, S, Se, Te, Po). Their acidic character increases from H2O to H2Te. The increase in acidic character can be explained in terms of decrease in bond enthalpy for the dissociation of H–E bond down the group. Owing to the decrease in enthalpy for the dissociation of H–E bond down the group, the thermal stability of hydrides also decreases from H2O to H2Po. All the hydrides except water possess reducing property and this character increases from H2S to H2Te.
-
Reactivity with oxygen: All these elements form oxides of the EO2 and EO3 types where E = S, Se, Te or Po. Ozone (O3) and sulphur dioxide (SO2) are gases while selenium dioxide (SeO2) is solid. Reducing property of dioxide decreases from SO2 to TeO2; SO2 is reducing while TeO2 is an oxidising agent. Besides EO2 type, sulphur, selenium and tellurium also form EO3 type oxides (SO3, SeO3, TeO3). Both types of oxides are acidic in nature
-
Reactivity towards the halogens: Elements of Group 16 form a large number of halides of the type, EX6, EX4 and EX2 where E is an element of the group and X is a halogen. The stability of the halides decreases in the order F– > Cl– > Br– > I–. Amongst hexahalides, hexafluorides are the only stable halides. All hexafluorides are gaseous in nature. They have octahedral structure. Sulphur hexafluoride, SF6 is exceptionally stable for steric reasons.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"