Thermal Expansion In Liquids And Gases MCQ - Practice Questions with Answers
Quick Facts
-
Thermal Expansion in liquids and gases is considered one the most difficult concept.
-
22 Questions around this concept.
Solve by difficulty
An ideal gas is initially at temp $T$ and volume $V$. Its volume is increased $\Delta V$ due to an increase in temperature $\Delta T$, with pressure remaining constant. The quantity $\delta=\frac{\Delta V}{V \Delta T}$ varies with temperature as
Concepts Covered - 1
Thermal Expansion in Liquids
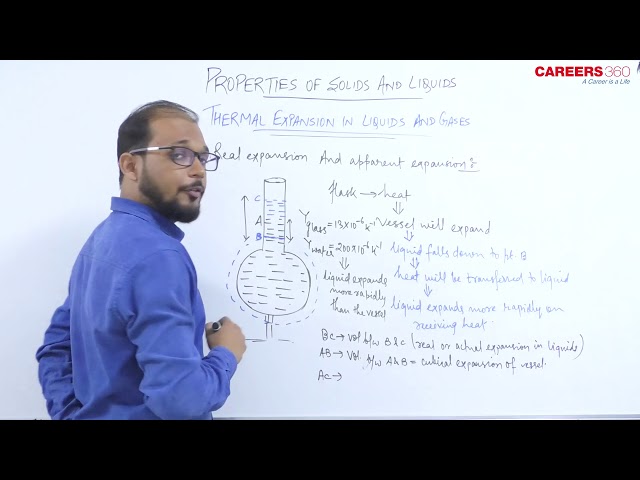
- Like solids, liquids do not have linear and superficial expansion but liquid only undergoes volume expansion.
- We always need some solid vessel to keep the liquid, so liquids are always to be heated along with a vessel which contains them so initially on heating the system (System is liquid + vessel here). Initially, the level of liquid in vessel falls (vessel expands more since it absorbs heat and liquid expands less) as the volume expansion co-effecient of solid is more than that of liquid but later on, it starts rising due to faster expansion of the liquid (because now solid transfer all the heat to liquid and that is the condition of steady state)
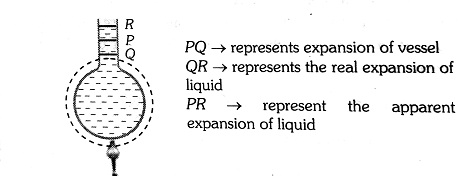
So, from above we can conclude that the actual increase in the volume of the liquid = The apparent increase in the volume of liquid + the increase in the volume of the vessel.
Basically liquids have two coefficients of volume expansion -
-
1. Co-efficient of apparent expansion $\gamma_a$ : It is due to an apparent (Apparent means that appears but not real) increase in the volume of liquid. This happens when the expansion of the vessel containing the liquid is not taken into account.
$$
\gamma_a=\frac{\text { Apparent expansion in volume }}{\text { Initial vdume } \times \Delta \theta}=\frac{(\Delta V)_o}{V \times \Delta \theta}
$$2. Co-efficient of real expansion $\gamma_r$ : This is due to the actual increase in the volume of liquid due to heating. In this expansion of vessel containing the liquid is taken into account.
$$
\gamma_r=\frac{\text { Real increase in volume }}{\text { Initial vdume } \times \Delta \theta}=\frac{(\Delta V)}{V \times \Delta \theta}
$$
Also coefficient of expansion of the flask $\gamma_{\text {vessel }}=\frac{\Delta V_{\text {vessel }}}{V \times \Delta \theta}$
So, $\gamma_{\text {Real }}=\gamma_{\text {Apporent }}+\gamma_{\text {Vessel }}$
So the change (apparent change) in volume in liquid relative to the vessel is -$$
\Delta V_{a p p}=V \gamma_{\text {app }} \Delta \theta=V\left(\gamma_{\text {Real }}-\gamma_{\text {Vessel }}\right) \Delta \theta=V\left(\gamma_r-3 \alpha\right) \Delta \theta
$$
Where, $\alpha=$ Coefficient of linear expansion of the vessel.
Anomalous expansion of water: Generally any material expands on heating and contracts on cooling. But in the case of water, it expands on heating if its temperature is greater than 4°C. In the range of 0°C to 4°C, water contracts on heating and expands on cooling, i.e. is negative. So water has this special property, which is not found in any existing natural material. This behavior of water in the range from 0°C to 4°C is called anomalous expansion. You can see it with the help of a graph.
This is the anomalous behaviour of water which causes ice to form first at the surface of a lake in cold weather. So, as winter approaches, the water temperature increases initially at the surface. It results in the water sinks because of its increased density. Consequently, the surface reaches 0°C first, and because of that the lake becomes covered with ice. This property of water makes the aquatic life survive the cold winter as the lake bottom remains unfrozen at a temperature of about 4°C.
At 4°C, the density of water is maximum while its specific volume is minimum.

Variation of Density with Temperature -
Most substances (solid and liquid) expand the heat supplied to them, i.e., the volume of a given mass of a substance increases on heating, so the density should decrease $\left(\right.$ as $\left.\rho \propto \frac{1}{V}\right)$. It means that the density is inversely proportional to the volume. From that we can deduce the expression of density after heating or cooling as follows -
$$
\begin{aligned}
& \frac{\rho^{\prime}}{\rho}=\frac{V}{V^{\prime \prime}}=\frac{V}{V+\Delta V}=\frac{V}{V+\gamma^{N \Delta \theta}}=\frac{1}{1+\gamma \Delta \theta} \\
& \Rightarrow \rho^{\prime}=\frac{\rho}{1+\gamma \Delta \theta}=\rho(1+\gamma \Delta \theta)^{-1}=\rho(1-\gamma \Delta \theta)
\end{aligned}
$$
Here, $\rho$ and $\rho^{\prime}$ is the density before and after heating the material
Expansion of Gases -
As we know gases have no definite shape. It takes the shape of the vessel in which it is kept. Therefore gases have only volume expansion. Since the expansion of the container (Because the container is solid) is negligible in comparison to the gases, therefore gases have only real expansion.
(1) Coefficient of volume expansion: At constant pressure, the unit volume of a given mass of a gas increases with a $1^{\circ} \mathrm{C}$ rise in temperature, which is called the coefficient of volume expansion.
$$
\alpha=\frac{\Delta V}{V_0} \times \frac{1}{\Delta \theta} \Rightarrow \text { Final volume } V^{\prime}=V(1+\alpha \Delta \theta)
$$
(2) Coefficient of pressure expansion:
$$
\beta=\frac{\Delta P}{P} \times \frac{1}{\Delta \theta}
$$
$\therefore$ Final pressure $P^{\prime}=P(1+\beta \Delta \theta)$
$\therefore$ Final pressure $P^{\prime}=P(1+\beta \Delta \theta)$
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"