Azeotropic Mixture MCQ - Practice Questions with Answers
Quick Facts
-
32 Questions around this concept.
Solve by difficulty
Among the following mixtures, dipole-dipole as the major interaction, is present in
According to Dalton's law, the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the pressures exerted by each gas if they occupy the same volume. Which of the following statements is true regarding Dalton's law?
Concepts Covered - 2
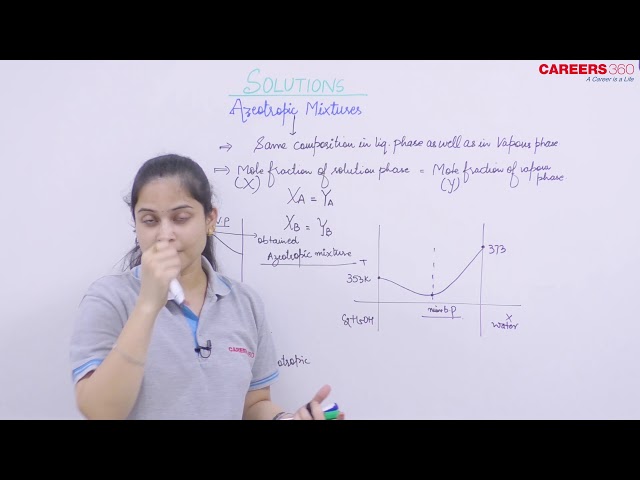
An azeotropic mixture is a mixture of two liquids having the same boiling point. These two liquids cannot be separated by simple distillation because of similar boiling point of the liquids. These mixtures are thus called constant boiling mixtures. These are formed by non-ideal solutions.
Azeotropic Mixture and Composition Curves
These are the mixture of two liquids which boils at one particular temperature like a pure liquid and distils over in the same composition that is, these are constant boiling mixtures. These are formed by non-ideal solutions. The study of the vapour pressure curves of such miscible liquids is of great help in separating the constituents of the liquid mixtures by distillation.
The separation by fractional distillation is possible only when the vapour phase has a composition different from that of the boiling liquid mixture.
Types of Azeotropic Mixtures
It is of the following types:
Azeotropic Mixtures with Minimum Boiling Point
The mixture of two liquids whose boiling point is less than either of the two pure components.
- This is formed by that composition of a non-ideal solution showing positive deviation for which the vapour pressure is maximum.
- Example, Ethanol (95.5%)+$ water (4.5%) mixture boils at 351.5 K.
- Such mixtures on distillation will give first fraction upto point M in pure state. After this the temperature will rise and the second component will pass over. Hence in such solutions also complete separation is not possible.

- The figure shows maximum vapour pressure at point M and therefore the solution has lowest boiling point.
Azeotropic Mixtures with Maximum Boiling Point
The mixtures of two liquids whose boiling point is more than either of the two pure components.
- This is formed by that composition of a non-ideal solution showing negative deviation for which the vapour pressure is minimum.
Example, HNO3(68%) + water(32%) mixture boils at 393.5 K.
Example, an aqueous solution of hydrochloric acid, when subjected to distillation, gives initially pure water and later forms a constant boiling mixture at 100oC which contains 20.24% acid. - In a mixture of two volatile liquids A and B, if A is more volatile and present in excess, then during distillation the vapours will be rich of component A and the liquid part will be richer in component B. Finally, we reach the point N where vapour pressure is minimum and the boiling point is maximum as shown in the figure.

- At this stage, the mixture distils unchanged in composition that is, complete separation of components from this type of solution into pure state is impossible.
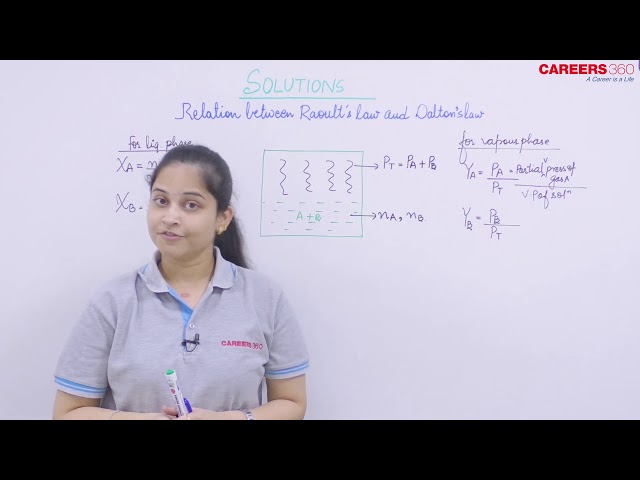
We have two liquids A and B and their vapour pressures are represented as PA and PB.
According to Raoult's law, we know:
Now, according to Dalton's law of partial pressure, we have:
Thus, on combining equations (i) with (iii) and (ii) with (iv), we get:
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"