Depression in Freezing Point MCQ - Practice Questions with Answers
Quick Facts
-
Depression in Freezing Point is considered one of the most asked concept.
-
30 Questions around this concept.
Solve by difficulty
What will happen when is added to aqueous solution
Choose the correct statement about freezing point:
Concepts Covered - 1
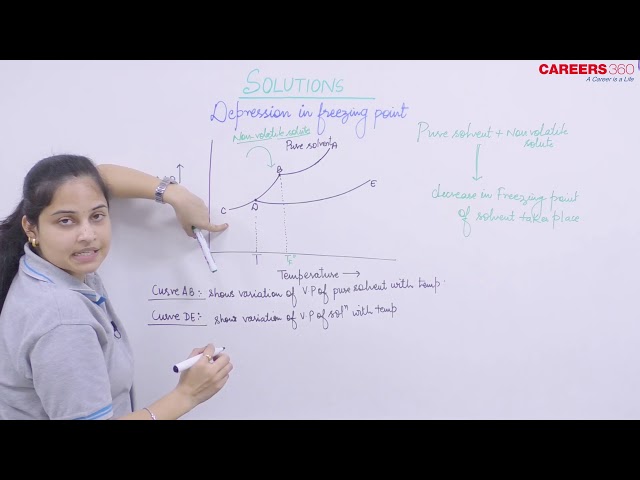
Freezing Point: It is the temperature at which the liquid and the solid form of the same substance are in equilibrium and have the same vapour pressure. A Solution freezes when it's vapour pressure is equal to the V.P of pure solid solvent. Due to lower vapour pressure of the solution, solid form of a solution separates out a lower temperature.
On adding a non-volatile solute to the solvent it's vapour pressure decreases and becomes equal to the V.P. of solid solvent at lower temperature hence the freezing point of solvent also decreases.
Suppose Tof Tf are the freezing point of pure solvent and solution respectively than decrease in freezing point ΔT is given as:

- This is also termed as cryoscopy and depression of freezing point (ΔTf)
- It can be measured by Beckmann's thermometer method and Rast's method.
- For a dilute solution, ΔTf is directly propotional to the molality (m) of the solution.
Hence ΔTf ∝ m
ΔTf = Kf m
If molality of the solution is one, then
ΔTf = Kf
ΔTf and M can be found out by using these relations.
NOTE: The value of Kv or Kf depends on only nature of solvent and not on the nature of the solute.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"