Vapour Pressure of solutions MCQ - Practice Questions with Answers
Quick Facts
-
68 Questions around this concept.
Solve by difficulty
The graph of vapour pressure and temperature for three different liquids X, Y, and Z is shown below:

The following inferences are made:
(A) X has higher intermolecular interactions compared to Y.
(B) X has lower intermolecular interactions compared to Y
(C) Z has lower intermolecular interactions compared to Y
The correct inference(s) is /are :
200 Ml at $\mathrm{O}_2(\mathrm{~g})$ from a Porous container in 200 sec .75 Ml at unknown gas effeuse under the Same condition of Temperature and pressure in 250 sec. Calculate vapour density of unknown gas.
The correct option for the value of vapour pressure of a solution at $45^{\circ} \mathrm{C}$ with benzene to octane in molar ratio 3:2 is :
[At $45^{\circ} \mathrm{C}$ vapour pressure of benzene is 280 mm Hg and that of octane is 420 mm Hg. Assume Ideal gas]
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
PA and PB are the vapour pressure of pure liquid components, A and B, respectively of an ideal binary solution. If XA represents the mole fraction of component A, the total pressure of the solution will be.
Concepts Covered - 4
It is the pressure exerted by vapours when in equilibrium with the liquid at a given temperature. It depends upon nature of liquid and temperature. Pure liquid has always a vapour pressure greater than its solution.
Vapour pressure of a liquid helps us to have an idea of forces of attraction amongst the molecules of liquid that is, more the force of attraction, lower is the vapour pressure and vice versa.
Vapour pressure of a liquid increase with an increase in temperature due to an increase in kinetic energy of solvent molecules that is, increase in evaporation however it is independent of the nature of the vessel.
Vapour Pressure of a Solution
When a miscible solute is added to a pure solvent, it results in the formation of solution. As some molecules of solute will replace the molecules of the solvent from the surface, therefore, escaping tendency of solvent molecules decreases. This causes a lowering of vapour pressure.
- The vapour pressure of a solution is less than that of pure solvent.
- If vapour pressure of solvent is P and that of solution is Ps then lowering of V.P = P - Ps.
- Vapour pressure of solution decreases as surface area occupied by solvent molecule decreases and density increases.
Vapour pressure depends on the following factors:
- Vapour pressure does not depend on the shape or size of the container.
- Vapour pressure depends on the nature of substance/liquid.
- Vapour pressure is inversely proportional to the force of attraction between molecules.
- Vapour pressure depends upon temperature. As temperature increases, evaporation increases and thus vapour pressure increases.
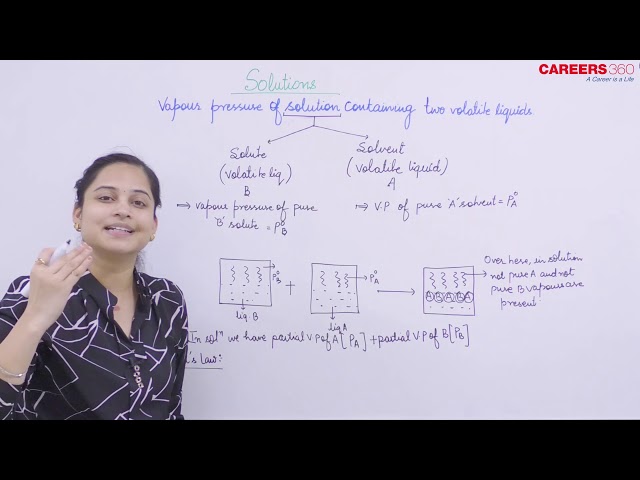
This is the solution when it has constituents i.e, solute and solvent as volatile. Lets represent solvent as "A" and solute as "B".
Now before mixing, vapour pressure of A is PoA and vapour pressure of B is PoB.
After mixing of solute and solvent, vapour pressure of solvent A and solute B will be partial pressures i.e, PA and PB.
Now according to Raoult's law, vapour pressure of liquid A is proportional to the mole fraction of liquid A.
Thus, PA = KXA and PB = KXB
Now, when we have only liquid A, then partial pressure of A is equal to PoA, thus K = PoA.
Thus, we can write:
PA = PoAXA and PB = PoBXB
Now according to Dalton's law of partial pressure, we have:
Total pressure(PT) = PA + PB
Thus, PT = PoAXA + PoBXB
The non-volatile solute is the solute which is present in solid-state. The solution is prepared by mixing of this solute in the liquid solvent.
Let's consider the solvent as "A" and solute as "B". Now, when no solute is present in the solvent, then the vapour pressure of the solvent is represented as PoA. Now when the solute is dissolved in the solvent then the vapour pressure of the solvent decreases and is represented as PA.
According to Raoult's law, we know:
PA = PoAXA .............(i)
Now, total mole fraction of solvent(XA) and solute(XB) is equal to 1.
Thus, XA + XB = 1
On putting the value of XA in equation (i), we get:
PA = PoA [1-XB]
PA = PoA - PoA XB
Therefore, PoA XB = PoA - PA
Thus, (PoA - PA) is also known as the lowering of vapour pressure
This equation is also known as 'Relative lowering of vapour pressure'.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"