Van't Hoff Factor and Abnormal Molar Mass MCQ - Practice Questions with Answers
Quick Facts
-
van't Hoff factor(i) or Abnormal Colligative Property is considered one the most difficult concept.
-
Calculation of Extent of Dissociation in an Electrolytic Solution is considered one of the most asked concept.
-
66 Questions around this concept.
Solve by difficulty
Which one of the following aqueous solutions will exhibit highest boiling point?
Of the following 0.10 aqueous solutions, which one will exhibit the largest freezing point depression?
If is the degree of dissociation of
the Van't Hoff’s factor (i) used for calculating the molecular mass is
NEET 2026: Application Form Link | Exam Centres List | How to Fill Form
NEET Prep: Mock Test | 10 Years PYQ's | Syllabus
NEET 2026: Boards Cheat Sheet | Mind Maps & Diagrams Guide | Formula Sheet
Latest: Allied and Health Sciences | Paramedical Universities Accepting Applications
Concepts Covered - 3
If a solute gets associated or dissociated in a solution, the actual number of particles are different from expected or theoretical consideration.
We know, that:
Thus, we can say that:
Again, we have:
Thus;
Suppose we have the solute A and it dissociates into B. Then the dissociation occurs as follows:
At time t = 0 1 0
At time t = t 1 - n
Thus, at time t = 0, initial number of solute particles = 1
And, at time t = t, observed number of solute particles = 1 - + n
= 1 + (n-1)
Thus, we know that:
where n = number of solute particles
= Degree of dissociation
NOTE: For strong electrolytes, = 1
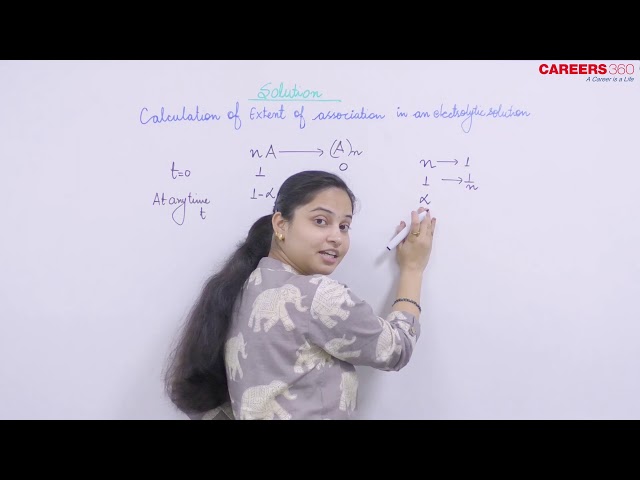
Suppose we have the solute A and it associates into (A)n. Then the association occurs as follows:
At time t = 0 1 0
At time t = t 1 -
/n
Now, initial number of solute particles = 1
And, observed number of solute particles = 1 - +
/n
= 1 + [1/n - 1]
Thus, van't Hoff factor is given as:
where, is the degree of association
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"