Thermodynamic Property MCQ - Practice Questions with Answers
Quick Facts
-
6 Questions around this concept.
Concepts Covered - 1
Properties of System
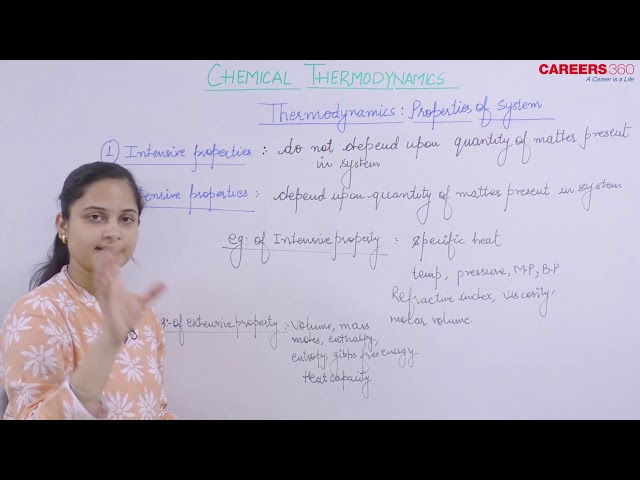
All macroscopic properties of a system irrespective of the fact whether they are state variable or not are divided into two types:
1. Intensive Properties
Such properties remains same on any division in system that is, do not depend upon the amount of substance present in the system.
Example: Temperature, pressure, concentration, density, viscosity, surface tension, specific heat, refractive index, pH, EMF of dry cell, vapour pressure dipole moment etc.
2. Extensive Properties
Such properties depend upon the amount of substance that is, their values are different in the divided system than in the entire system
Example, Mass, volume, energy, work, internal energy, entropy, enthalpy, heat capacity, length.
An extensive property can be made intensive specifying it In unit amount of matter.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"