Planck's quantum theory MCQ - Practice Questions with Answers
Edited By admin | Updated on Sep 25, 2023 25:23 PM | #NEET
Quick Facts
-
13 Questions around this concept.
Solve by difficulty
The energy required to break one mole of bonds in
. The longest wave-length of light capable of breaking a single
bond is
Concepts Covered - 1
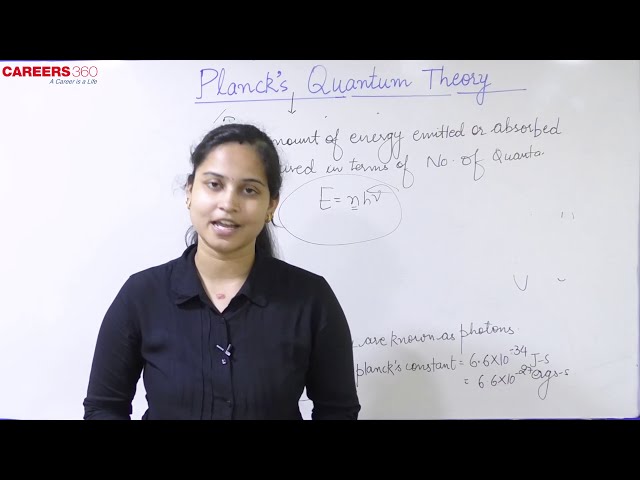
Planck’s quantum theory
Atom and molecules could emit (or absorb) energy only in discrete quantities known as quanta and not in a continuous manner.
-
The nature of emission of radiation from hot bodies (black -body radiation).
-
Ejection of electrons from a metal surface when radiation strikes it (photoelectric effect).
-
Variation of heat capacity of solids as a function of temperature.
-
Line spectra of atoms with special reference to hydrogen.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"