Chemical Equilibrium MCQ - NEET Practice Questions with Answers
Quick Facts
-
18 Questions around this concept.
Solve by difficulty
If the value of an equilibrium constant for a particular reaction is then at equilibrium the system will contain
Concepts Covered - 2
The word "equilibrium" in a physical sense is explained as the "No change of state of the body". When the two opposing processes (reaction) occur simultaneously with equal rates, the system is in the state of equilibrium. Equilibrium is classified as follows

When an equilibrium exists between the same chemical species, it is called physical equilibrium. For example:
When an equilibrium exists between different chemical species, it is called chemical equilibrium.
If a chemical equilibrium has only one phase, it is called homogenous and if more than one phase it is called heterogeneous.
Chemical Equilibrium
"It is the state of a reversible reaction at which measurable properties like colour, density, pressure concentration are nearly unchangeable.
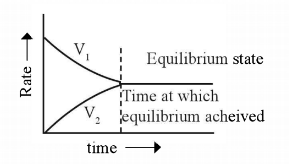
Here V1 and V2 are the rates of forward and backward reactions respectively. i.e, equilibrium is the state in a reversible reaction at which the rate of forward and backward reactions or two opposing reactions are same.
Types of reactions
Chemical reactions are of two types:
Irreversible Reaction: Such reactions occur in one direction only and get completed.
For example:
(i) When unreactive products or solid products are formed.
(ii) All precipitate reactions are irreversible.
(iii) Neutralisation reactions are also irreversible.
(iv) Redox reactions are also irreversible.
(v) Combustion reactions are also irreversible.
Reversible Reactions: Such reactions occur in both directions i.e., forward and backward direction however never complete as the products can give back the reactants under same or different conditions. For example:
-
Vapourization of water in open flask is irreversible reaction while in closed flask it is reversible.
-
Decomposition of CaCO3 in open flask is irreversible reaction while in closed flask it is reversible.
The following are the important characteristics of equilibrium:
- It is obtained only when the reversible reaction is carried out in a closed space.
- Here the rate of forward reaction is equal to rate of backward reaction.
- Here both forward and backward reactions are taking place with same rate hence the relative amounts of the reactants and products present at equilibrium does not change with time.
- At constant temperature, it is characterized by properties like colour, density, pressure etc.
- It is possible from both sides.
- It is dynamic in nature. It means the reaction or process is not going to be ceased as reaction occurs in both directions with equal rates.
- A catalyst cannot alter the positive of equilibrium as it accelerates both the forward and backward reactions to the same extent this means the same state of equilibrium is reaction i.e., a positive catalyst can set up equilibrium in less time but can not change it.
- At equilibrium 𝚫G is equal to zero i.e.,
𝚫G = 𝚫H - T𝚫S
So,
𝚫H = T𝚫S - Under the similar conditions of temperature, concentration and pressure, the same state of equilibrium is reached.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"















