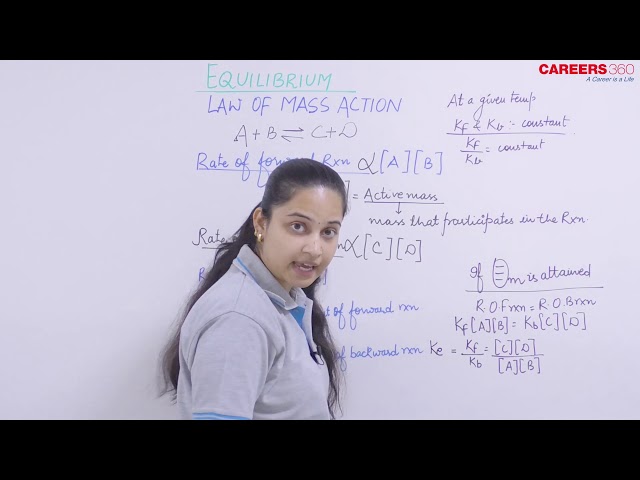Law Of Mass Action MCQ - Practice Questions with Answers
Quick Facts
-
3 Questions around this concept.
Solve by difficulty
In a 25 L container, 15 moles of $\mathrm{N}_2$ reacted with 12 moles of $\mathrm{H}_2$ at 800 K . It was observed that 6 moles of $\mathrm{H}_2$ was present after equilibrium. The gaseous mixture was suddenly cooled to 300 K after addition of 4.35 L of $\mathrm{H}_2 \mathrm{O}(l)$ in the mixture. What will be the final pressure of the mixture keeping in mind the following points.
- All $\mathrm{NH}_3$ dissolved in water.
- There is no change in volume of the liquid.
- No reaction took place between $\mathrm{N}_2$ and $\mathrm{H}_2$ at 300 K
Also, neglect the V.P. of the liquid solution.

Concepts Covered - 1
It was introduced by Guldberg and Waage. It states that “the rate at which a substance reacts is directly proportional to its active mass and the rate at which substances react together is directly proportional to the product of their active masses each raised to a power which is equal to the corresponding stoichiometric number the substance present in the chemical reaction".
If active masses of A, B, C and D are [A], [B], [C] and [D] respectively, then:
Rate of reaction of A ∝ [A]
Rate of reaction of B ∝ [B]
V1 ∝ [A] [B] = K1 [A][B]
V2 ∝ [C] [D] = K2 [C][D]
NOTE: Law of mass action is not applicable for solids as for them active mass is always one.
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"