pH Of Acids And Bases MCQ - Practice Questions with Answers
Quick Facts
-
pH of Solutions: Strong Acids, pH of Solutions: Weak Acids, pH of solution/mixture are considered the most difficult concepts.
-
pH of Solutions: Strong Bases are considered the most asked concepts.
-
18 Questions around this concept.
Solve by difficulty
Which one of the following statements is not true?
Among the following acids which has the lowest value?
In a buffer solution containing an equal concentration of and
, the
is
. The pH of the buffer solution is:
Which of the following salts will give highest pH in water?
Concepts Covered - 4
pH is also referred to as potential or power of hydrogen. Mathematically, it can be represented as follows:
If solution is neutral, then:
Kw = [H3O+][OH-]
From the ionic product of water, we know:
Kw = 10-14
[H3O+] = [OH-] = x (since solution is neutral)
Thus, 10-14 = Kw = x2
x = 10-7
Now, [H3O+] = 10-7
Thus, pH = - log10(H3O+) = - log10(10-7) = 7
For Acidic solutions: For Basic solutions:
For acidic solutions, we must have [H3O+] > [OH-] For basic solutions, we must have [H3O+] < [OH-]
Thus, [H3O+] > 10-7 Thus, [H3O+] < 10-7
Thus, [H3O+] for acids can be 10-6, 10-5, 10-4, etc. Thus, [H3O+] for basics can be 10-8, 10-9, 10-10, etc.
Thus, pH of acids can be 6, 5, 4, etc. Thus, pH of basics can be 8, 9, 10, 11, etc.
Hence, pH of acidic solutions is less than 7 Hence, pH of basic solutions is greater than 7
pH depends upon temperature
We know from ionic product of water that at 630C, the value of Kw = 10-13.
For neutral solution we know:
Hence, pH depends upon temperature
pH of Strong Acids
Strong acids are those acids which dissociate completely in solutions. For example:
- 2 x 10-3 M HNO3
Since HNO3 is a strong acid, thus it will dissociate completely into H+ and OH- ions as follows:
Thus, pH of HNO3 is 2.7
- 10-4 M H2SO4
Since H2SO4 is a strong acid, thus it will dissociate completely into H+ and OH- ions as follows:
Thus, pH of H2SO4 is 3.7
NOTE: If molarity(N) of solution is not given but normality(N) is given, then molarity can be calculated using the following formula:
N = M x n
where, n is the number of moles
pH of Weak Acids
Weak acids are those acids which dissociate partially in solutions. For example:
- 8 M HA (Ka =2 x 10-8)
The chemical equation for the dissociation of weak acid HA is as follows:
Initial: 8M 0 0
Equil: 8 - 8𝛂 8𝛂 8𝛂
The equilibrium constant Ka for the weak acid is given as follows:
Thus, pH of this given acid = 3.4
- 0.002N CH3COOH(𝛂 = 0.02)
The chemical equation for the dissociation of CH3COOH is as follows:
Initial: c 0 0
Equil: c - c𝛂 c𝛂 c𝛂
The equilibrium constant Ka for the weak acid is given as follows:
Thus, pH of acetic acid = 4.4
Strong bases
Strong bases are those bases which dissociate completely in solution. For exaple:
- 0.1M NaOH
Since NaOH is a strong base, thus it will dissociate completely into Na+ and OH- ions. The chemical equation for the dissociation of NaOH is as follows:
Hence, pH of 0.1M NaOH solution is 13.
- 0.05M Ba(OH)2
Since Ba(OH)2 is a strong base, thus it will dissociate completely into Ba2+ and 2OH- ions. The chemical equation for the dissociation of Ba(OH)2 is as follows:
Hence, pH of 0.05M Ba(OH)2 solution is 13.
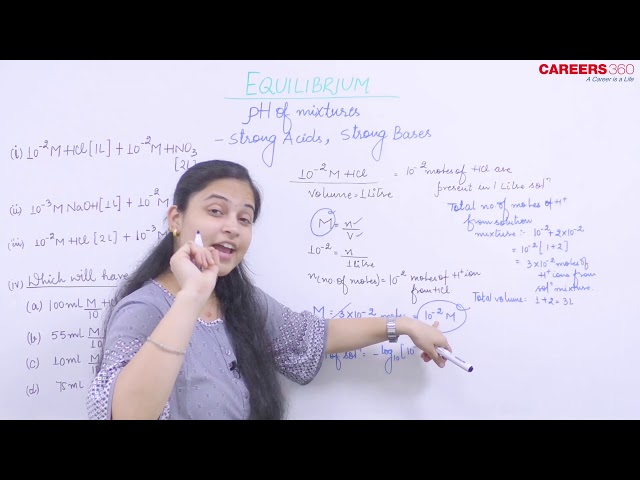
- Mixture of Strong Acids:
NOTE: Shortcut only for monobasic acids and monoacidic bases:
- Mixture of Strong Bases:
Using the shortcut formula for bases as given above, we get:
- Mixture of Strong Acid and Strong Base:
Clearly, moles of (H+) = 2 x 10-2 moles and moles of (OH-) = 1 x 10-3 moles.
Since moles of (H+) is greater than moles of (OH-), therefore the solution medium will be acidic.
Now, remaining moles of H+ = 2 x 10-2 - 10-3 = 19 x 10-3
Thus, pH of the mixture = 2.2
Study it with Videos
"Stay in the loop. Receive exam news, study resources, and expert advice!"