Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
The AIIMS Paramedical entrance exam is one of the popular exams for admission to paramedical courses in AIIMS Institutes of India. One of the most effective ways to prepare for the AIIMS Paramedical Nursing entrance exam is by practising the AIIMS Paramedical question papers. Using the sample and previous year’s AIIMS paramedical question papers, students can get a good idea of the types of questions, how hard they are, and the overall format of the exam. By working through these papers and checking the solutions, candidates can improve their speed, accuracy, and confidence for the real test.
This Story also Contains

The AIIMS Paramedical Nursing Exam comprises 90 multiple-choice questions from Physics, Chemistry, and Biology. As there is no in-depth syllabus provided by AIIMS, the previous year's AIIMS Paramedical paper serves as a source to note down key topics and most-asked questions. Practising with solved papers on a regular basis also helps in proper revision and time management during the test.
Students looking for AIIMS Paramedical admission should practice question papers of AIIMS Paramedical to evaluate their study progress by attempting past years’ question papers. The previous year question paper of AIIMS Paramedical exam helps students understand the exam format, the kinds of questions asked, and how tough the actual exam might be.
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Question Paper | Download PDF |
AIIMS Paramedical Nursing Question Paper | Will be provided |
1. Bicarbonate ion is produced inside:
a) Basophils
b) Erythrocytes
c) Neutrophils
d) Lymphocytes
Answer: (b) Erythrocytes
Explanation: In erythrocytes, which are also known as red blood cells is the cell is where bicarbonate ion production takes place. In the cell, some carbon dioxide binds to haemoglobin, forming carbaminoglobin. The carbonic anhydrase enzyme present in red blood cells quickly converts carbon dioxide and water into bicarbonate and hydrogen ions. So the correct answer is 'Erythrocytes'.
2. Reduction in the pH of blood will:
a) Release bicarbonate ions by the liver
b) Decrease the affinity of haemoglobin with oxygen
c) Reduce the blood supply to the brain
d) Reduce the rate of heartbeat
Answer: (b) Decrease the affinity of haemoglobin with oxygen
Explanation: Reduction in pH of blood causes oxygen haemoglobin dissociation curve to shift to the right, which indicates dissociation of oxygen from haemoglobin. This decreases the affinity of haemoglobin for oxygen.
3. The amount of air exchanged in breathing can be measured with a:
a) Barometer
b) Sphygmomanometer
c) Spherometer
d) Spirometer
Answer: (d) Spirometer
Explanation: Spirometry is the process of recording the changes in the volume movement of air into and out of the lungs, and the instrument used for the purpose is called a spirometer or respirometer.
4. For reaching left side of heart, blood must pass through:
a) Kidneys
c) Lungs
b) Liver
d) Brain
Answer: (c) Lungs
Explanation: The deoxygenated blood pumped into the pulmonary artery is passed on to the lungs, from where the oxygenated blood is carried by the pulmonary veins into the left atrium. Hence, for reaching the left side of the heart, blood must pass through the lungs.
5. The Bowman’s capsule is:
a) A part of the nephron, and is the site of filtration of various blood constituents during the formation of urine
b) A part of the uriniferous tubule and is the site of absorption of salt and glucose
c) A part of the nephron and is the site of secretion of various blood constituents during the formation of urine
d) A part of the uriniferous tubule and is the site of reabsorption-sorption of water and glucose
Answer: (a) A part of nephron and is the site of filtration of various blood constituents during the formation of urine
6. Kidney crystals are solid clusters of:
a) Calcium nitrate and uric acid
b) Calcium carbonate and uric acid
c) Calcium metabisulfite and uric acid
d) Phosphate and uric acid
Answer: (d) Phosphate and uric acid
7. The pelvic girdle of man consists of:
Ilium, ischium and pubis
Coracoid, scapula and clavicle
Ilium, ischium and coracoid
Ilium, coracoid and scapula
Answer: (a) Ilium, ischium and pubis
Explanation: The pelvic girdle consists of two coxal bones and each coxal bone is formed by the fusion of three bones - ilium, ischium and pubis. At the point of fusion of the above bones is a cavity called the acetabulum to which the thigh bone articulates.
8. The nerves leading to the central nervous system are called:
a) Afferent
b) Efferent
c) Motor
d) Thoracic nerves
Answer: (a) Afferent
9. Stimulated blood flow and higher blood sugar level results when the following hormone rises in concentration:
a) Oxytocin
b) Vasopressin
c) Glucagon
d) Epinephrine
Answer: (d) Epinephrine
10. Hormones are:
Secretions of all glands
All enzymes
Chemical messenger
Excretory substances
Answer: c) Chemical messenger
1. The molar mass of C6H10O5 is:
a) 172.09 u
b) 182 g
c) 152.00 g/mol
d) 162.14 g/mol
Answer: (d) 162.14 g/mol
Explanation: Molar mass of C6H10O5 is as follows:
= [(atomic mass of C) × 6] + [(atomic mass of H ) × 10] + [ (atomic mass of O) × 5]
= [(12.0107 × 6) + (1.008 × 10) + (15.9994 × 5)]
= 162.1406 g/mol
2. The first ionisation potential of Na is 5.1 eV. The value of the electron gain enthalpy of Na + would be:
a) - 5.1 eV
b) - 2.55 eV
c) - 10.2 eV
d) 2.55 eV
Answer: a) -5.1 eV
3. Most favourable conditions for electrovalent (ionic) bonding are:
a) High ionisation potential of one atom and low electron affinity of the other atom
b) Low electron affinity and low ionisation potential of both the atoms
c) Low ionisation potential of one atom and high electron affinity of the other atom
d) High electron affinity and high ionisation potential of both the atoms
Answer: (c) Low ionisation potential of one atom and high electron affinity of the other atom
Explanation: Low ionisation energy of the element forming a cation ε1 high electron affinity of the element forming an anion.
4. Which bond is expected to be the least polar?
a) Si - N
b) B - Cl
c) O - F
d) P - F
Answer: (c) O-F
5. Chlorobenzene is
Nearly as reactive as benzyl chloride
Less reactive than benzyl chloride
More reactive than any alkyl halide
More reactive than benzyl chloride
Answer: (b) less reactive than benzyl chloride
6. Identify the correct reaction from the following.
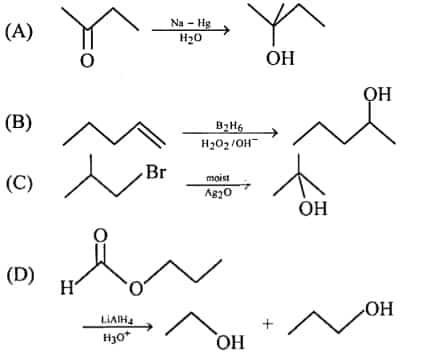
Answer: (c)
7. Which of the following will not react with Na metal?
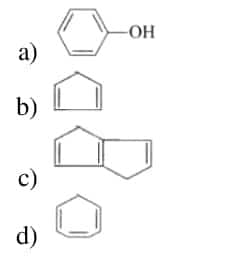
Answer: (d)
8. Mole fraction of the solute in a 1.00 molal aqueous solution is:
a) 0.0344
b) 0.0177
c) 0.1770
d) 1.7700
Answer: (b) 0.0177
9. Pick up an incorrect statement about the given structure
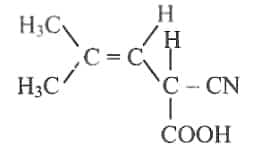
The chiral centre shows optical activity
Number of optical isomers = 2
It shows geometrical isomerism
Number of chiral centres = 1
Answer: (c) It shows geometrical isomerism
10: If an excess of AgNO 3 solution is added to 100 ml of a 0.024 M solution of dichlorobis ethylenediamine Cobalt (III) chloride, how many moles of AgCl will be precipitated?
a) 0.0012
c) 0.0048
b) 0.0024
d) 0.0016
Answer: (b) 0.0024
1. In the SI system, the fundamental units are
a) Meter, kilogram, second, ampere, Kelvin, mole and watt
b) Meter, Newton, second, ampere, Kelvin, mole and candela
c) Meter, kilogram, second, coulomb, Kelvin, mole, candela and horse power
d) Meter, kilogram, second, ampere, Kelvin, mole and candela
Answer: (d) meter, kilogram, second, ampere, Kelvin, mole and candela
Explanation: The SI base units and their physical quantities are the metre for measurement of length, the kilogram for mass, the second for time, the ampere for electric current, the kelvin for temperature, the candela for luminous intensity, and the mole for amount of substance.
2. A cylindrical wire of mass (0.4 ± 0.01) g has length (8 ± 0.04) cm and radius (6 ± .03) mm. The maximum error in its density will be
a) 5%
b) 1%
c) 4%
d) 3.5%
Answer: c) 4%
3. A bus starts from a depot with a constant acceleration of 0.20 m/s2 . A passenger arrives at depot 8 s after the end of the bus left the very same point. What is the least constant speed at which the passenger can run and catch the bus?
a) 1.6 m/s
b) 4.2 m/s
c) 2.8 m/s
d) 3.2 m/s
Answer: d) 3.2 m/s
4. A particle moves in x - y plane according to the rule x = a sinω t and y = a cos ω t. The particle follows:
a) An elliptical path
b) A straight line path inclined equally to x and y axes
c) A parabolic path
d) A circular path
Answer: d) A circular path
5. Passengers standing in a bus are thrown outwards when the bus takes a sudden turn. This happens because of:
a) Outward pull on them
b) Inertia
c) Change in momentum
d) Change in acceleration
Answer: (b) inertia
Explanation: Due to the law of inertia, the upper part of the body continues moving in the direction of the initial velocity while the lower part comes into motion along the direction of the new velocity.
6. A moving mass of 8 kg collides elastically with a stationary mass of 2 kg. If E is the initial kinetic energy of the moving mass, the kinetic energy left with it after the collision will be:
a) 0.36 E
b) 0.08 E
c) 0.64 E
d) 0.80 E
Answer: a) 0.36 E
7. Two blocks of masses 5 kg and 2 kg are placed on a frictionless surface and connected by a spring. An external kick gives a velocity of 14 m/sec to the heavier block in the direction of the lighter one. Velocities of two blocks in the centre of mass frame, just after the kick, are respectively given by:
a) 10 m/s, - 10 m/s
b) 10 m/s, 4 m/s
c) 4 m/s, 10 m/s
d) 4 m/s, - 10 m/s
Answer: (d) 4 m/s, -10 m/s
Explanation: The centre of mass reference frame is one in which the centre of mass is at rest. So, the velocity of the heavier block in this frame just after the kick is,
v'1 = v1 - VCM = 14 - 10 = 4 m/s and that of the lighter block is v'2 - v2 - VCM = 0 - 10 = -10 m/s.
8. A moving coil galvanometer has a resistance 50Ω and it indicates full deflection at 4ma current. A voltmeter is made using this galvanometer and a 5 kΩ resistance. The maximum voltage that can be measured using this voltmeter, will be close to
a) 10 V
b) 15 V
c) 20 V
d) 40 V
Answer: c) 20 V
9. If a spherical mirror is immersed in a liquid, its focal length will be:
Depending on the nature of the liquid
Increase
Decrease
Remain unchanged
Answer: d) Remain unchanged
10. Magnetism in substances is caused by [1]
Hidden magnets
Orbital motion of electrons only
Due to spin and orbital motions of electrons both
Spin motion of electrons only
Answer: (c) due to spin and orbital motions of electrons both
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly
Recognized as Category 1 University by UGC | Accredited with A+ Grade by NAAC | Scholarships available