Thermodynamics NEET, Solutions To Question Papers Of Last Six Years
A good rank in the National Eligibility cum Entrance Test (NEET) for undergraduates is a dream of many students who wish to get an MBBS seat in colleges across India. To prepare for NEET, students should practice the previous year's questions and understand the important areas to be focused on for the NEET exam. Every question of NEET is important as it is a highly competitive exam. Here we analyse the Class 12 NCERT chapter Thermodynamics using past six-year papers and discuss the past six-year questions from Thermodynamics NEET Physics paper.

In the last six years of NEET papers a total of eight questions were asked from the chapter Thermodynamics. Thermodynamics belongs to the Class 11 NCERT Physics syllabus. NEET Thermodynamics is a small chapter that can be understood easily. Questions from Thermal Properties Of Matter are not included here. Only the NCERT Class 11 Physics, chapter 12 - Thermodynamics is included. Let us see the topics covered in the past six years of NEET papers and their weightage.
Topics And Number Of Questions
Topic | Number Of Questions | Weightage |
First Law Of Thermodynamics | 2 | 25% |
Thermodynamic Processes | 4 | 50% |
Carnot Engine | 2 | 25% |
A total of eight questions are analysed out of which three are from thermodynamic processes. From the topic thermodynamic process, two questions are from adiabatic processes and one is a match to the following questions that cover all the important thermodynamic processes. Two questions were from the topics First Law Of Thermodynamics and Carnot Engine. Let us have a look at the previous year questions from Thermodynamics (2017-2022).
Also Read| NEET Previous Year Question Papers
Q 1 - NEET 2017
A Carnot engine having an efficiency of 1/10as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at a lower temperature is:
Answer-
For a Carnot cycle
![]()
![]() = Coefficient of performance
= Coefficient of performance
![]() = Efficiency of Carnot engine
= Efficiency of Carnot engine
![]()
![]()
![]()
Q 2 - 2017
Thermodynamic processes are indicated in the following diagram.
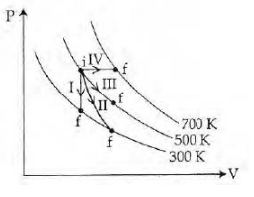
Match the following
Column-1 Column-2
| P. Process I | a. Adiabatic |
| Q. Process II | b. Isobaric |
| R. Process III | c. Isochoric |
| S. Process IV | d. Isothermal |
Answer-
P ![]() c, Q
c, Q ![]() a, R
a, R ![]() d, S
d, S ![]() b
b
Process I-volume is constant-isochoric
Process IV-pressure is constant-Isobaric
Process II is adiabatic and Process III is isothermal
Q 3 - NEET 2018
A sample of 0.1 g of water at 100°C and normal pressure (1.013 x 105 Nm-2) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample, is
Answer-
![]()
![]()
![]()
Change in internal energy-
![]()
Q 4 - NEET 2018
The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
Answer-
![]()
![]()
Q 5 - 2018
The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is
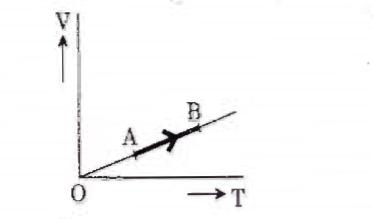
Answer-work done =
![]()
heat absorbed = ![]()
![]()
ratio of work to heat
![]()
Q 6 - 2019
In which processes, the heat is neither absorbed nor released by a system?
Answer-
In the adiabatic process heat is neither absorbed nor released by the system.
Q 7 - 2021
Two cylinders A and B of equal capacity are connected to each other via a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stop cock is suddenly opened. The process is:
Answer-
As the entire system is thermally insulated and as free expansion will be taking place the temperature of the gas remains the same. The process is adiabatic
A similar type of question is asked in the NCERT Class 11 physics book. The question is given below-
Two cylinders A and B of equal capacity are connected to each other via a stopcock. A contains gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stopcock is suddenly opened. Answer the following
(a) What is the final pressure of the gas in A and B ?
(b) What is the change in internal energy of the gas?
(c) What is the change in the temperature of the gas?
(d) Do the intermediate states of the system (before settling to the final equilibrium state) lie on its P-V-T surface?
Q 8- 2022
An ideal gas follows a process described by ![]() from
from ![]() to
to ![]() (C is a constant). Then
(C is a constant). Then
if P1 > P2 then T2 > T1
if V2 > V1 then T2 < T1
if V2 > V1 then T2 > T1
if P1 > P2 then V2 > V1
![]()
![]()
TV = constant
so
![]()
All the concepts of NCERT Class 11 chapter Thermodynamics are essential for the NEET exam. Following the NCERT book and the previous year, question papers will give enough information to crack the NEET questions from Thermodynamics.
Popular Courses and Specializations
List of colleges accepting NEET
Browse Medicine Colleges by State
Questions related to NEET
On Question asked by student community
Ideally you should be able to. However, this is a minor detail and doesnt change much so we would advise and see if its an editable field. If its not, please donot worry. This detail is not of much concern and should not have any bearing in your admission as
Yes, it is an entrance exam. Please register at http://www.upvetuniv.edu.in/ .
Prospectus can be found at https://upvetuniv.edu.in/wp-content/uploads/2026/02/Prospectus-2026-27Final.pdf
You can check the NEET PYQs by Careers360 for previosu years' question papers.
Yes, You can change the date of birth in the NEET registration correction window.
To know what are the details can be corrected in the submitted NEET application form, Click here .
Yes — you can sit for NEET (National Eligibility cum Entrance Test) even if you took the Commerce stream in school, provided you meet the eligibility criteria set by the National Medical Commission (NMC) and your state/university. Key points and steps:
Eligibility criteria (core requirements)
-
Academic subjects: You must have
Begin a career in Medical and Allied Sciences. Admissions Open for
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Amity University-Noida Health and Allied Sciences Admissions
ApplyAmongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
SRM Kattankulathur Dental College Admissions 2026
ApplyRanked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
SRM Medical College Admissions 2026
ApplyRanked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly




