JSS University Mysore 2025
NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs
Important Chemistry Formulas for NEET: Are you studying for the NEET UG 2025 examination and looking to get admission in one of the best medical colleges in India? Chemistry is among the most important subjects in NEET, and a lot of questions are actually formula-based. Familiarity with these formulas can help you solve questions faster and with greater accuracy. Since the NEET 2025 exam is on 4th May 2025, now is the ideal time to practice and tighten your hold on important concepts of Physical, Organic, and Inorganic Chemistry.
In this article, topic-wise, the NEET Chemistry Formula Sheet PDF 2025 is compiled, containing all the major equations and reactions you should memorize. These formulas are carefully chosen to make sure that aspirants score better in the exam with smart effort. Be it last-minute revision or practicing mock tests, this formula sheet will improve speed, accuracy, and confidence. Download the PDF and improve NEET Chemistry preparation today!
Given below are the important formulas for chemistry, divided as per the topics. Students can go through these to improve their chances of scoring better in NEET 2025 Exam.
NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs
Admissions open for Bachelor of Physiotherapy, B.Sc Nutrition & Dietetics ,B.Sc Food Science & Technology
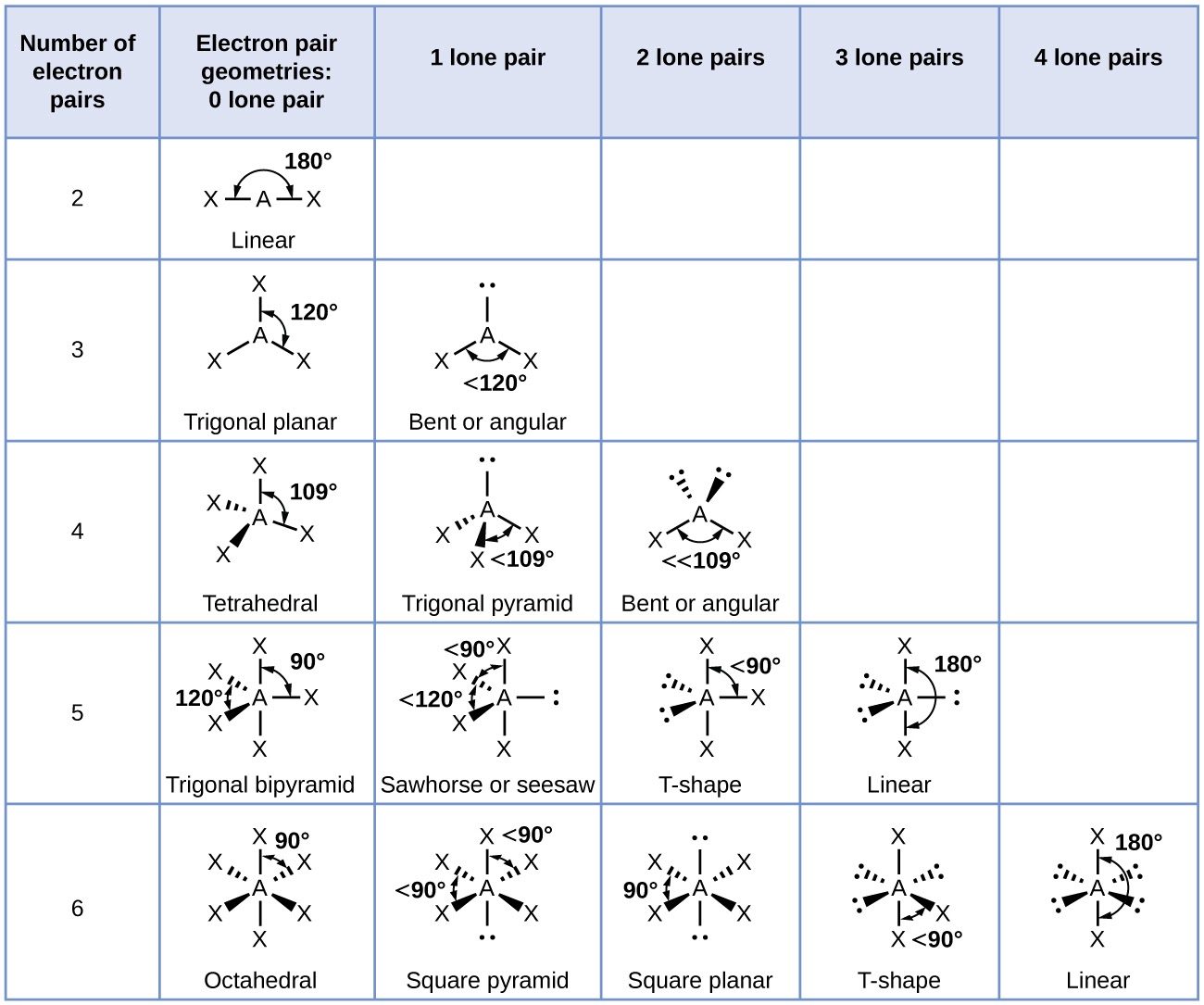
The ideal shapes of molecules, which are predicted based on electron pairs and lone pairs of electrons, are mentioned in the table below:
General Representation
AₓBᵧ ⇌ xAʸ⁺ + yBˣ⁻ Ksp = [Aʸ⁺]ˣ × [Bˣ⁻]ʸ
Relation between Solubility (s) and Solubility Product (Ksp):
AₓBᵧ ⇌ xAʸ⁺ + yBˣ⁻ (s → xs, ys)
Ksp = xˣ yʸ sˣ⁺ʸ
Also read:
Boyle’s law may be expressed mathematically as:
P ∝ 1/V (at constant T and n)
or
V ∝ 1/P (at constant T and n)
Where:
T = temperature
P = pressure of the gas
n = number of moles of the gas
V = volume of the gas
⇒ V = k1 × 1/P
Here, k₁ is the proportionality constant whose value depends upon the following factors.
Alternate Forms of Boyle’s Law
P1V1 = P2V2 = constant
or
PV = K1
or
P1/P2 = V2/V1
P₁ / P₂ = V₂ / V₁
Coordination Number (C. No.)
In Simple Cubic (SC): 6
In Face Centered Cubic (FCC): 12
In Body Centered Cubic (BCC): 8
Density of Lattice Matter (d)
It is the ratio of mass per unit cell to the total volume of a unit cell, and it is found out as follows.
d=Z × Atomic weight N0 × Volume of unit cell (a3)
Here, d = Density
Z = Number of atoms
N0 = Avogadro number
a3 = Volume
a = Edge length
To find the density of a unit cell in cm3, m must be taken in g/mole and should be in cm.
It is the ratio of the radius of an octahedral void to the radius of the sphere forming the close-packed arrangement. Normally, ionic solids are more compact, as voids are also occupied by cations (which are smaller in size). The pattern of arrangements and type of voids both depend on the relative size (ionic size) of two ions in a solid.
For example, when r+ = r-, the most probable and favourable arrangement is the BCC type.
With the help of relative ionic radii, it is easier to predict the most probable arrangement. This property is expressed as a radio ratio.
Radius ratio =r + (radius of cation )r − (Radius of anion)
From the value of the radius ratio, it is clear that the larger the radius ratio, the larger the size of the cation, and the more anions needed to surround it — that is, the more coordination numbers.
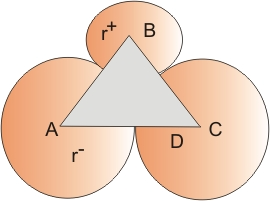
Radius ratio for tetrahedron
Angle ABC is the tetrahedral angle of 109.5∘
∠ABD = 109.52 = 5475
In triangle ABD
Sin ABD = 0.8164 = AD/AB
or r∗ + rr = 10.8164 = 1.225
or r∗r = 0.225
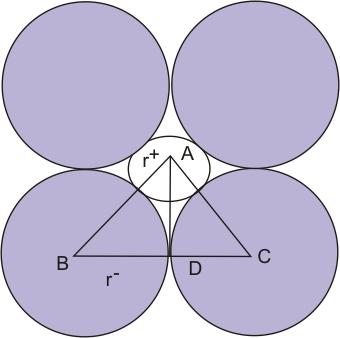
Radius ratio for octahedron
AB = r⁺ + r
BD = r
∠ABC = 45°
In triangle ABD:
Cos(∠ABD) = 0.7071 = BD / AB = r / (r⁺ + r)
So,
(r⁺ + r) / r = 1 / 0.7071 = 1.414
Therefore,
r⁺ / r = 1.414 − 1 = 0.414
Colloidal particles always carry an electric charge. The nature of this charge is the same for all the particles in a given colloidal solution and may be either positive or negative.
An interesting feature in the variability of oxidation states of the d-block elements is noticed among the groups. Although in the p–block the lower oxidation states are favoured by the heavier members (due to the inert pair effect), the opposite is true in the groups of d-block.
Also read:
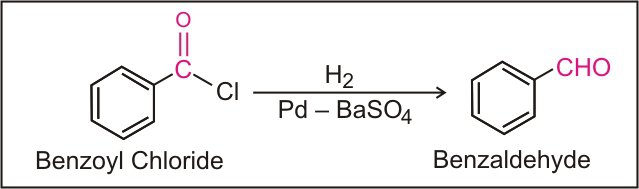
Rosenmund Reduction
Stephen Reduction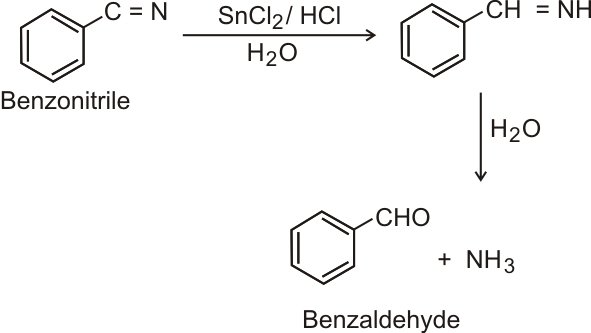
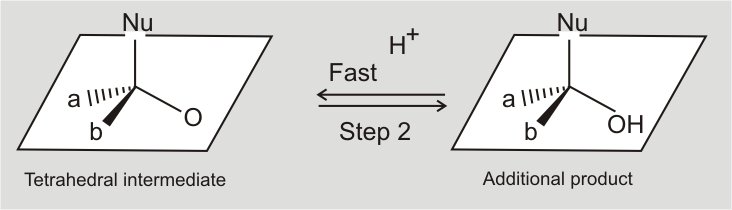
(i) Mechanism of nucleophilic addition reactions: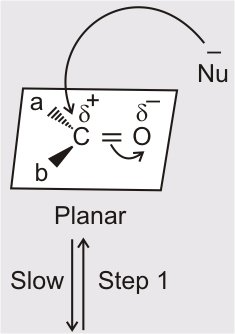
Reduction to hydrocarbons
The carbonyl group of aldehydes and ketones is reduced to the CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid (Clemmensen reduction) hydrazine hydrazone or with hydrazine, followed by heating with sodium or potassium hydroxide in a high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).
Oxidation
Aldehydes differ from ketones in their oxidation reactions. Aldehydes are easily oxidised to carboxylic acids on treatment with common oxidising agents like nitric acid, potassium permanganate, potassium dichromate, etc. Even mild oxidising agents, mainly Tollens’ reagent and Fehlings’ reagent, also oxidise aldehydes.
Formation of Anhydride
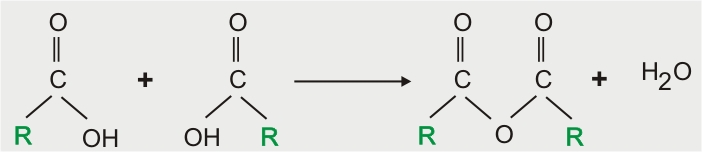
Reaction with Ammonia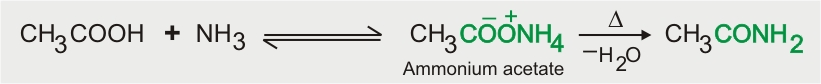
Reduction
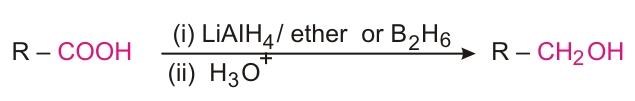
Decarboxylation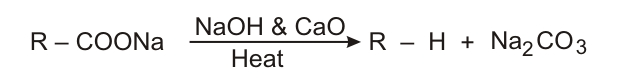
Kolbe's electrolysis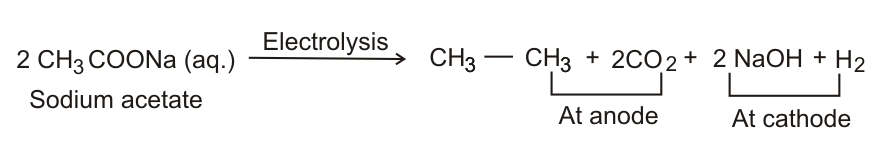 Halogenation
Halogenation 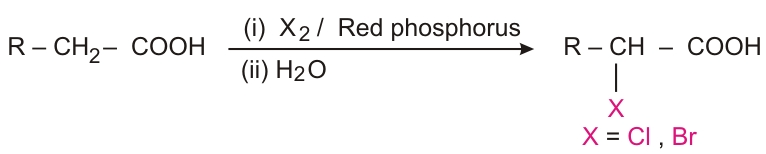
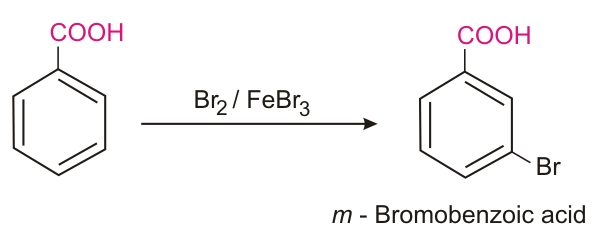
Ring substitution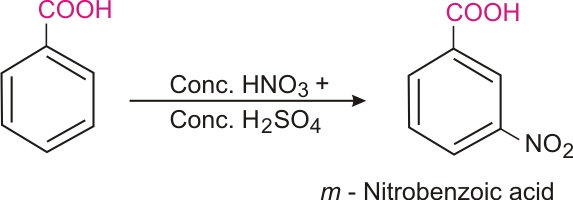
Carbohydrates -
These are polyhydroxy aldehydes, ketones or substances that form these on hydrolysis and possess at least one chiral atom. The (-OH) group is available in the form of hemiacetals or hemiketals.
Carbohydrates can be classified into three categories:
Monosaccharides: They are the simplest carbohydrates, which cannot be hydrolysed into smaller molecules. They are sweet and crystalline and are called sugars.
Oligosaccharides: These carbohydrates, on hydrolysis, give two to nine molecules of monosaccharides classified as di-, tri, tetra-saccharides, etc. For example, sucrose, maltose, lactose, raffinose, etc. They are also called sugars.
Polysaccharides: These carbohydrates, on hydrolysis, give a large number of monosaccharides, e.g., starch, cellulose, etc. They are also called non-sugars.
Reducing and Non-Reducing Sugars
Those sugars which reduce Fehling's and Tollens's solutions are called reducing sugars and those which do not reduce these reagents are called non-reducing sugars.
Also Read:
Application Date:08 April,2025 - 07 May,2025
Application Date:16 April,2025 - 06 May,2025
Sometimes, the official website might be experiencing heavy traffic, especially right after the admit cards are released. This can cause errors or make the website slow to respond. A slow internet connection on your end can also prevent the admit card from downloading properly. In some cases, browser extensions like ad-blockers might interfere with the download process. It's also possible that you might be entering the wrong application number, password, or security pin.
Here are a few things you can try to fix this issue. First, double-check that you are entering your NEET application number, password, and the security pin correctly. It might be helpful to copy and paste these details directly from your registration confirmation if you have it. If the website is slow, try refreshing the page or try downloading it again after some time, perhaps during off-peak hours like late at night or early morning.
Try using a different web browser like Google Chrome or Mozilla Firefox. Also, clear your current browser's cache and cookies or try downloading the admit card in incognito or private browsing mode. If you have any browser extensions enabled, especially ad-blockers or VPNs, try temporarily disabling them and then attempt to download the admit card again. Ensure that you have a stable and good-speed internet connection. Avoid using mobile data hotspots if possible and prefer a broadband or Wi-Fi connection. If you've tried all of this and still can't download the admit card, try using a different device like another computer or a mobile phone.
If none of these steps work, it's important to contact the National Testing Agency (NTA) for help. You can reach them through their helpline numbers: 011-40759000 or 011-69227700. You can also send them an email at neet@nta.ac.in. They should be able to assist you with the issue. Remember to keep trying and don't panic. There's still time before the exam, and the NTA will likely help you resolve this.
I hope this helps.
The National Testing Agency (NTA), which conducts the NEET exam, usually provides a few options to recover your password if you've forgotten it. You'll need to visit the official NEET website, which is typically neet.nta.nic.in. On the login page where you'd normally enter your application number and password, you should find a link that says something like "Forgot Password?"
Clicking on this "Forgot Password?" link will generally present you with a few methods to reset your password. One common method involves answering a security question that you would have set up during the NEET registration process. If you remember the answer to your chosen security question, you can enter it, and the website will likely allow you to set a new password.
Another frequent option is to receive a One-Time Password (OTP) on your registered mobile number or email address. If you choose this method, you'll likely need to enter your application number and date of birth. The NTA will then send an OTP to your registered contact details. Once you receive and enter this OTP on the website, you should be able to create a new password. In some cases, there might also be an option to reset your password via a link sent to your registered email address. If you select this, the NTA will email you a link. Clicking on this link will redirect you to a page where you can set a new password for your NEET account.
After you've successfully reset your password using any of these methods, make sure to note it down in a safe place for future reference. Once you have your new password, go back to the admit card download section on the official NEET website, enter your application number, your newly recovered password, and the security pin displayed on the screen. You should then be able to log in and download your NEET admit card.
If you encounter any difficulties during this process, or if none of these options seem to be working, it's best to directly contact the NTA for assistance. You can find their helpline numbers and email address on the official NEET website under the "Contact Us" section. They will be the most reliable source to help you resolve any login issues and access your admit card.
Predicting specific questions with 95% certainty for NEET 2025 is impossible. The National Testing Agency (NTA) designs the paper to test a broad understanding of the Class 11 and 12 NCERT syllabi for Physics, Chemistry, and Biology. However, based on past trends, important concepts, and high-weightage chapters, we can identify areas that are highly likely to have questions.
In Physics, expect a significant number of questions from:
In Chemistry, focus on:
In Biology (Botany and Zoology), emphasize:
I hope this helps.
Yes, candidates with low vision are permitted to use assistive devices, such as video magnifiers, during the NEET UG 2025 examination. The National Testing Agency (NTA) allows Persons with Benchmark Disabilities (PwBD) to use their own assistive devices, provided they have a valid PwD certificate issued by a recognized medical authority. Additionally, PwBD candidates are entitled to extra time (1 hour and 5 minutes) and the option to use a scribe, depending on their specific needs. It is advisable to inform the examination authorities in advance and carry relevant medical certificates to facilitate the use of such devices during the exam
Yes, a low vision candidate is allowed to use assistive devices like a video magnifier to read the NEET UG 2025 question paper, provided they meet certain conditions.
Benchmark Disability : Must have 40% or more disability, certified by a recognized authority.
Medical Certificate : Submit a certificate stating the need for the device.
NTA Approval : Get prior approval from NTA to use the device during the exam.
Extra Time : Eligible for 60 minutes of extra time.
Make sure to follow the application guidelines and seek NTA approval well in advance.
Orthotists and Prosthetists are professionals who provide aid to patients with disabilities. They fix them to artificial limbs (prosthetics) and help them to regain stability. There are times when people lose their limbs in an accident. In some other occasions, they are born without a limb or orthopaedic impairment. Orthotists and prosthetists play a crucial role in their lives with fixing them to assistive devices and provide mobility.
A career in pathology in India is filled with several responsibilities as it is a medical branch and affects human lives. The demand for pathologists has been increasing over the past few years as people are getting more aware of different diseases. Not only that, but an increase in population and lifestyle changes have also contributed to the increase in a pathologist’s demand. The pathology careers provide an extremely huge number of opportunities and if you want to be a part of the medical field you can consider being a pathologist. If you want to know more about a career in pathology in India then continue reading this article.
A veterinary doctor is a professional, working in animal healthcare. He or she conducts medical examinations, diagnoses, and treats various illnesses of animals. Animals have distinct internal organs and functions, requiring specialised attention from a veterinary doctor. A doctor who treats humans cannot offer the same level of care to animals due to these variations. Therefore, a veterinary doctor plays a critical role in animal welfare.
Veterinary professionals prevent illness by providing vaccines and offering advice on animal nutrition and overall health. Their knowledge extends beyond household animals and includes livestock, wildlife, and exotic animals. Individuals who love animals and want to treat their illnesses, injuries, and diseases must opt for a career as a veterinary doctor.
Speech therapists are essential medical professionals addressing speech disorders. Whether it's delayed speech in children or difficulties in pronunciation, these experts play a crucial role. This article explores how to become a speech therapist in India, covering courses, colleges, and the responsibilities of this impactful profession.
Gynaecology can be defined as the study of the female body. The job outlook for gynaecology is excellent since there is evergreen demand for one because of their responsibility of dealing with not only women’s health but also fertility and pregnancy issues. Although most women prefer to have a women obstetrician gynaecologist as their doctor, men also explore a career as a gynaecologist and there are ample amounts of male doctors in the field who are gynaecologists and aid women during delivery and childbirth.
The audiologist career involves audiology professionals who are responsible to treat hearing loss and proactively preventing the relevant damage. Individuals who opt for a career as an audiologist use various testing strategies with the aim to determine if someone has a normal sensitivity to sounds or not. After the identification of hearing loss, a hearing doctor is required to determine which sections of the hearing are affected, to what extent they are affected, and where the wound causing the hearing loss is found. As soon as the hearing loss is identified, the patients are provided with recommendations for interventions and rehabilitation such as hearing aids, cochlear implants, and appropriate medical referrals. While audiology is a branch of science that studies and researches hearing, balance, and related disorders.
An oncologist is a specialised doctor responsible for providing medical care to patients diagnosed with cancer. He or she uses several therapies to control the cancer and its effect on the human body such as chemotherapy, immunotherapy, radiation therapy and biopsy. An oncologist designs a treatment plan based on a pathology report after diagnosing the type of cancer and where it is spreading inside the body.
Are you searching for an ‘Anatomist job description’? An Anatomist is a research professional who applies the laws of biological science to determine the ability of bodies of various living organisms including animals and humans to regenerate the damaged or destroyed organs. If you want to know what does an anatomist do, then read the entire article, where we will answer all your questions.
A Narcotics Officer is an officer employed by the state to investigate the usage of drugs and their trafficking. A narcotics officer conducts undercover operations, investigates suspected drug dealers, executes raids and other appropriate actions for arresting these traffickers to reduce the circulation of drugs in the country.
A narcotics officer works in collaboration with other government agencies to stop drug trafficking at borders. He or she engages with various NGOs and public organisations to teach people about the dangerous effects of drug usage. A narcotics officer plays an important role in reducing the illegal activities of drug dealers and the circulation of drugs in the nation.
If we talk about a career as a research associate, it all comes down to one thing - curiosity towards nature and the passion to find answers. A career as a research associate is full of thrill and excitement. However, a research associate also faces a lot of challenges and failures while working on a project. A job of a research associate includes a spectrum of Science as a subject in detail.
A career as a Drug Inspector is regarded as one of the most diverse in the field of healthcare and pharmacy. Candidates must undergo a screening process administered by the UPSC and or SPSCs in order to become drug inspectors. Those who manage it through the selection process will have a rewarding career with a high salary.
A Biotechnologist is a professional who possesses strong knowledge and techniques that are utilised in creating and developing innovative products that improve the quality of human life standards. A biochemist uses biological organisms to create and improve goods and procedures for agriculture, medicine, and sustainability. He or she researches the genetic, chemical, and physical characteristics of cells, tissues, and organisms to determine how they can be used industrially.
A career as R&D Personnel requires researching, planning, and implementing new programs and protocols into their organization and overseeing new products’ development. He or she uses his or her creative abilities to improve the existing products as per the requirements of the target market.

NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs

Accorded Institution of Eminence by MoE, Govt. of India | NAAC A++ Grade | Ranked #4 India by NIRF 2024

Accorded Institution of Eminence by MoE, Govt. of India | NAAC A++ Grade | Ranked #4 India by NIRF 2024
Know possible Govt/Private MBBS/BDS Colleges based on your NEET rank
Register for Careers360 NEET Counseling & Admission Guidance Service.
Apply for Integrated M.Sc Biotechnology (5 Years, after 10+2) @ VIT Bhopal University | H-CTC 52 LPA