Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Important NEET Formulas for Physics and Chemistry are helpful for scoring well in the exam. NEET is a highly competitive test where speed and accuracy matter the most. Having all key formulas at fingertips saves time during problem-solving. The NEET formula also helps in quick revisions before the exam. A strong understanding of formulas ensures that students can focus more on applying concepts rather than recalling them.
This Story also Contains

In Physics, formulas related to mechanics, electrostatics, thermodynamics, and Modern Physics are repeatedly asked. In Chemistry, formulas from physical chemistry, such as the mole concept, thermodynamics, equilibrium, and electrochemistry, play a major role. Learning these formulas in a structured way increases confidence and reduces mistakes. Thus, mastering NEET Physics and Chemistry formulas is an important step in NEET exam preparation.
| Important Physics Formula for NEET 2026 | Download Here |
The important physics formulas for NEET based on the NEET exam trends observed in the previous years are provided here. The table consists of the highest-scoring concepts in the last 5 years, the number of times they appeared in NEET, and the years in which questions were based on these concepts. The physics NEET 2026 formula sheet is based on these high-scoring concepts.
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
|
S. No. |
Concept title |
Chapter |
Number of times appeared in the Previous year's papers (last 6 years) |
|
1. |
Series LCR circuit |
Electromagnetic Induction and Alternating Currents |
6 |
|
2. |
Equations of motion of SHM |
Oscillations and Waves |
7 |
|
3. |
Electric potential due to continuous charge distribution (II) |
Electrostatics |
5 |
|
4. |
Resistance and Resistivity |
Current Electricity |
6 |
|
5. |
Parallel Grouping of Resistance |
Current Electricity |
4 |
|
6. |
Kirchhoff's second law |
Current Electricity |
4 |
|
7. |
Nature of Electromagnetic Waves |
Electromagnetic Waves |
4 |
|
8. |
Total Internal Reflection |
Optics |
4 |
|
9. |
Young's double slit experiment -1 |
Optics |
4 |
|
10. |
De-Broglie wavelength of an electron |
Dual Nature of Matter and Radiation |
4 |
|
11. |
Logic Gates |
Electronic devices |
7 |
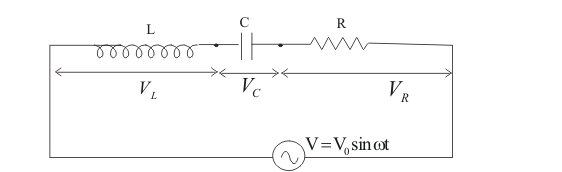
Let i be the amount of current in the circuit at any time, and VL, VC, and VR the potential drops across L, C, and R, respectively.
$v_R = iR \Rightarrow$ Voltage is in phase with $i$,
$v_L = i \omega L \Rightarrow$ Voltage leads $i$ by $90^{\circ}$,
$v_C = i \omega C \Rightarrow$ Voltage lags $i$ by $90^{\circ}$.
By all these, we can draw a phasor diagram as shown below –
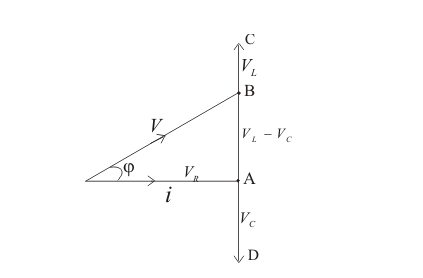
So, from the above phasor diagram, V will represent the resultant of vectors VR and (VL−VC).
So the equation becomes –
$V = \sqrt{V_R^2 + (V_L - V_C)^2} = \sqrt{(iR)^2 + (X_L - X_C)^2} = \sqrt{(iR)^2 + \left( \omega L - \frac{1}{\omega C} \right)^2} = iZ,$
where $Z = \sqrt{R^2 + \left( \omega L - \frac{1}{\omega C} \right)^2}$ is called the impedance of the circuit.
Also, $\tan \phi = \frac{V_L - V_C}{V_R} = \frac{X_L - X_C}{R} = \frac{\omega L - \frac{1}{\omega C}}{R}.$
As we know, $a = -\omega^2 x$
General equation of SHM
1. For Displacement:
$x = A \sin(\omega t + \phi)$ ; where $\phi$ is the initial phase and $(\omega t + \phi)$ is called the phase.
Various displacement equations:
(1) $x = A \sin \omega t \Rightarrow$ when the particle starts from mean position towards right.
(2) $x = -A \sin \omega t \Rightarrow$ when the particle starts from mean position towards left.
(3) $x = A \cos \omega t \Rightarrow$ when the particle starts from extreme position towards left.
(4) $x = -A \cos \omega t \Rightarrow$ when the particle starts from left extreme position towards right.
2. For Velocity ($v$):
$x = A \sin(\omega t + \phi) \Rightarrow v = \frac{dx}{dt} = A \omega \cos(\omega t + \phi) = A \omega \sin\left(\omega t + \phi + \frac{\pi}{2}\right)$
3. For Acceleration ($a$):
$x = A \sin(\omega t + \phi) \Rightarrow v = \frac{dx}{dt} = A \omega \cos(\omega t + \phi) = A \omega \sin\left(\omega t + \phi + \frac{\pi}{2}\right)$
$\Rightarrow a = \frac{dv}{dt} = -A \omega^2 \sin(\omega t + \phi) = A \omega^2 \sin\left(\omega t + \phi + \pi\right) = -\omega^2 x$
So, the phase difference between $x$ and $v$ is $\frac{\pi}{2}$.
Similarly, the phase difference between $v$ and $a$ is $\frac{\pi}{2}$.
And, the phase difference between $a$ and $x$ is $\pi$.
Differential equation of SHM:
$\frac{dv}{dt} = -\omega^2 x \Rightarrow \frac{d}{dt}\left(\frac{dx}{dt}\right) = -\omega^2 x \Rightarrow \frac{d^2x}{dt^2} + \omega^2 x = 0$
If the motion of any particle satisfies this equation, then that particle performs Simple Harmonic Motion (SHM).
Based on the NEET 2026 exam pattern seen over the previous five years, we have assembled the important chemistry formulas for the NEET exam. The concepts that have scored the highest over the past five years, along with the number of times they have appeared and the years in which questions based on them have been asked, are listed in the table below. The NEET chemistry formula listed below is based on these high-scoring concepts:
|
S. No. |
concept title |
Chapter |
Number of times appeared in the Previous year's paper (last 6 years) |
|
1. |
Shapes of Molecules |
Chemical Bonding and Molecular Structure |
3 |
|
2. |
Solubility and Solubility Products |
Equilibrium |
3 |
|
3. |
The Gas Laws- Boyle’s Law (Pressure- Volume Relationship) |
States of Matter: Gases and Liquids |
3 |
|
4. |
Mathematical Analysis of Cubic System |
Solid state |
4 |
|
5. |
Charge on Colloids |
Surface Chemistry |
3 |
|
6. |
Oxidation State |
d and f Block Elements |
3 |
|
7. |
Preparation of Aldehydes |
Aldehydes, Ketones and Carboxylic Acids |
4 |
|
8. |
Nucleophilic Addition Reaction |
Aldehydes, Ketones and Carboxylic Acids |
5 |
|
9. |
Reduction and Oxidation reactions |
Aldehydes, Ketones and Carboxylic Acids |
4 |
|
10. |
Chemical Properties of Carboxylic Acids |
Aldehydes, Ketones and Carboxylic Acids |
3 |
|
11. |
Carbohydrates |
Biomolecules |
3 |
Aspirants can download the important NEET chemistry formula from the table given below:
| NEET Chemistry Formula 2026 | Download Here |
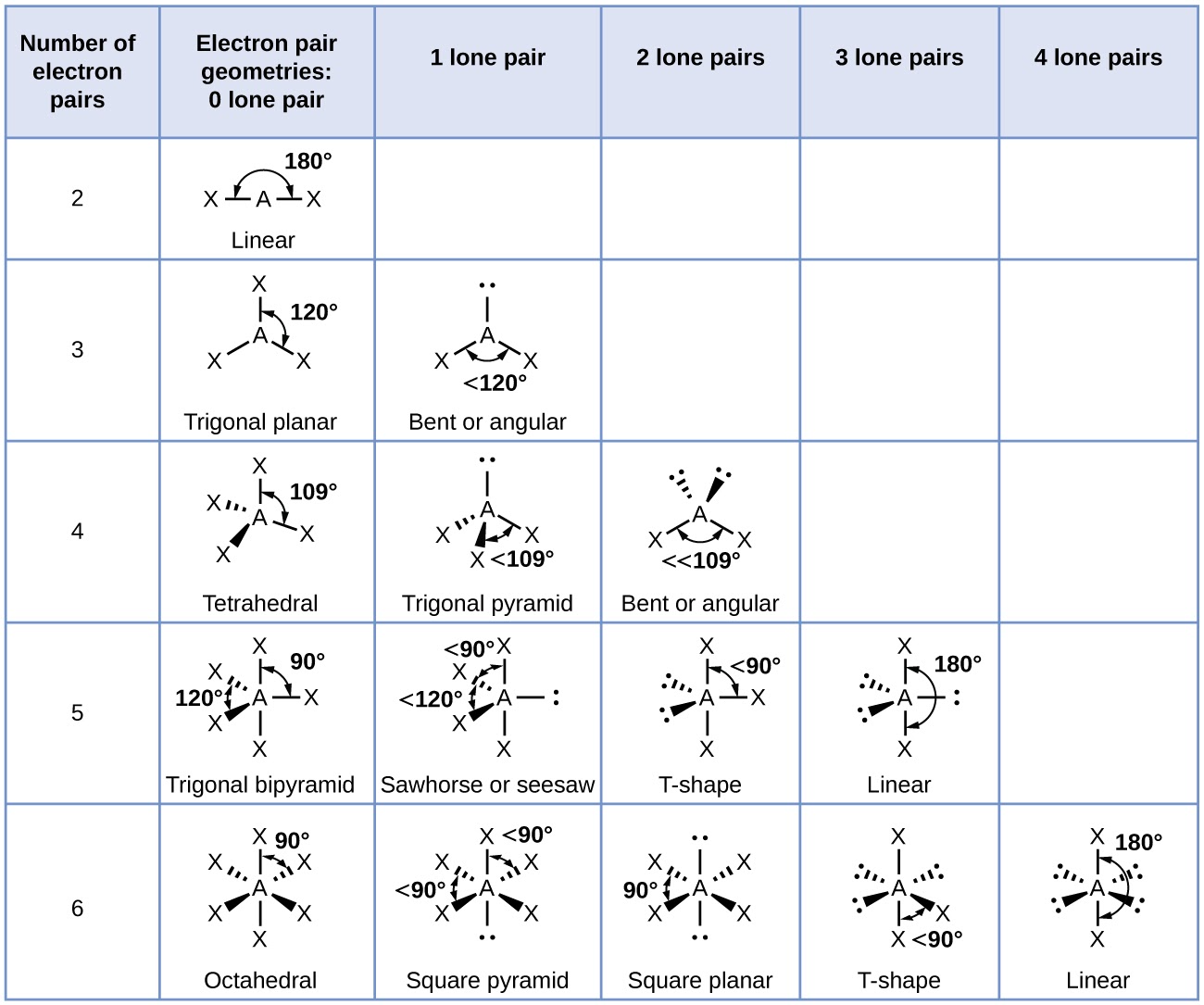
The ideal shapes of molecules, which are predicted based on electron pairs and lone pairs of electrons, are mentioned in the table below:
General Representation:
$A_xB_y \rightleftharpoons xA^{+} + yB^{-}$
$K_{sp} = [A^{+}]^x [B^{-}]^y$
Relation between Solubility ($s$) and Solubility Product ($K_{sp}$):
$A_xB_y \rightleftharpoons xA^{+} + yB^{-}$
If solubility is $s$, then $[A^{+}] = x s$ and $[B^{-}] = y s$
Thus,
$K_{sp} = (x s)^x (y s)^y = x^x y^y s^{x + y}$
The Gas Laws — Boyle’s Law (Pressure–Volume Relationship):
$P \propto \frac{1}{V} \Rightarrow PV = k \; (\text{at constant } T)$
On Question asked by student community
Ideally you should be able to. However, this is a minor detail and doesnt change much so we would advise and see if its an editable field. If its not, please donot worry. This detail is not of much concern and should not have any bearing in your admission as
Yes, it is an entrance exam. Please register at http://www.upvetuniv.edu.in/ .
Prospectus can be found at https://upvetuniv.edu.in/wp-content/uploads/2026/02/Prospectus-2026-27Final.pdf
If you have uploaded only the left and right thumb impressions instead of all the required fingers in the NEET UG 2026 application form , you should know that the NTA does not allow candidates to edit biometric details, such as finger and thumb impressions, during the correction window. You
Hello student,
Please go through our article to get your question answered. The article gives information on admission with 300 marks in NEET 2026.
Link - How to get admission with 300 marks in NEET 2026?
Hope this is helpful!
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly