JSS University Mysore 2025
NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs
33 Questions around this concept.
For the solution of the gases, w,x,y and z in water at 298 K, the henry's law constants (KH) are 0.5,2,35 and 40 kbar, respectively. The correct plot for the given data is
What is the solubility of oxygen gas in water at $25^{\circ} \mathrm{C}$ and a partial pressure of 1.0 atm , according to Henry's Law?
At 298 K, the solubility of oxygen gas in water is .
The concentration of oxygen gas in equilibrium with the air is 0.21 atm. What is the Henry's law constant for oxygen gas in water?
Calculate the molar solubility of oxygen in water if exerts partial pressure of
in vapour above water
Find the pressure of gas required to dissolve in 48gm gas in 200gm liquid if 600gm liquid consists of 18gm ethane at any temperature T, at pressure 3atm.
Which of the following is NOT a real-life example of Henry's Law?
Why is it difficult to remove dissolved oxygen from water using boiling or heating?
Which of the following statements is NOT true regarding the application of Henry's Law in scuba diving?
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature. It depends upon the nature of solute and solvent as well as temperature and pressure. Let us consider the effect of these factors in solution of a solid or a gas in a liquid.
Solubility of a Solid in a Liquid
Every solid does not dissolve in a given liquid. While sodium chloride and sugar dissolve readily in water, naphthalene and anthracene do not. On the other hand, naphthalene and anthracene dissolve readily in benzene but sodium chloride and sugar do not. It is observed that polar solutes dissolve in polar solvents and non polar solutes in non-polar solvents. In general, a solute dissolves in a solvent if the intermolecular interactions are similar in the two or we may say like dissolves like.
When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is known as dissolution. Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallisation. A stage is reached when the two processes occur at the same rate. Under such conditions, number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
At this stage the concentration of solute in solution will remain constant under the given conditions, i.e., temperature and pressure. Similar process is followed when gases are dissolved in liquid solvents. Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution. An unsaturated solution is one in which more solute can be dissolved at the same temperature. The solution which is in dynamic equilibrium with undissolved solute is the saturated solution and contains the maximum amount of solute dissolved in a given amount of solvent. Thus, the concentration of solute in such a solution is its solubility.
Earlier we have observed that solubility of one substance into another depends on the nature of the substances. In addition to these variables, two other parameters, i.e., temperature and pressure also control this phenomenon.
Effect of temperature: The solubility of a solid in a liquid is significantly affected by temperature changes. Consider the equilibrium represented by the equation. This, being dynamic equilibrium, must follow Le Chateliers Principle. In general, if in a nearly saturated solution, the dissolution process is endothermic (∆sol H > 0), the solubility should increase with rise in temperature and if it is exothermic (∆sol H < 0) the solubility should decrease. These trends are also observed experimentally.
Effect of pressure: Pressure does not have any significant effect on solubility of solids in liquids. It is so because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.
Solubility of a Gas in a Liquid
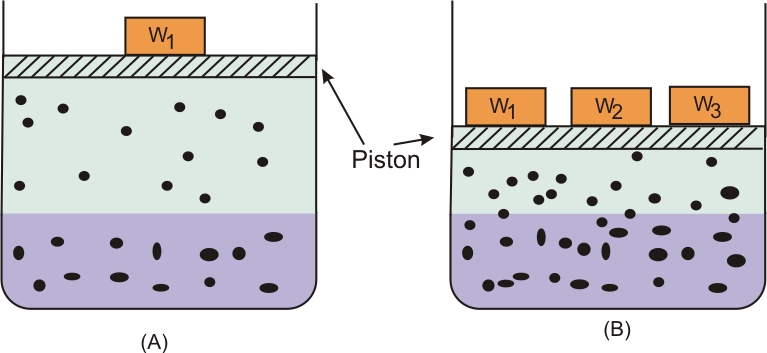
Many gases dissolve in water. Oxygen dissolves only to a small extent in water. It is this dissolved oxygen which sustains all aquatic life. On the other hand, hydrogen chloride gas (HCl) is highly soluble in water. Solubility of gases in liquids is greatly affected by pressure and temperature. The solubility of gases increase with increase of pressure. For solution of gases in a solvent, consider a system as shown in figure given below. The lower part is solution and the upper part is gaseous system at pressure p and temperature T. Assume this system to be in a state of dynamic equilibrium, i.e., under these conditions rate of gaseous particles entering and leaving the solution phase is the same. Now increase the pressure over the solution phase by compressing the gas to a smaller volume. This will increase the number of gaseous particles per unit volume over the solution and also the rate at which the gaseous particles are striking the surface of solution to enter it. The solubility of the gas will increase until a new equilibrium is reached resulting in an increase in the pressure of a gas above the solution and thus its solubility increases.
Henry was the first to give a quantitative relation between pressure and solubility of a gas in a solvent which is known as Henry’s law. The law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution. Dalton, a contemporary of Henry, also concluded independently that the solubility of a gas in a liquid solution is a function of partial pressure of the gas. If we use the mole fraction of a gas in the solution as a measure of its solubility, then it can be said that the mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution. The most commonly used form of Henry’s law states that “the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution” and is expressed as:
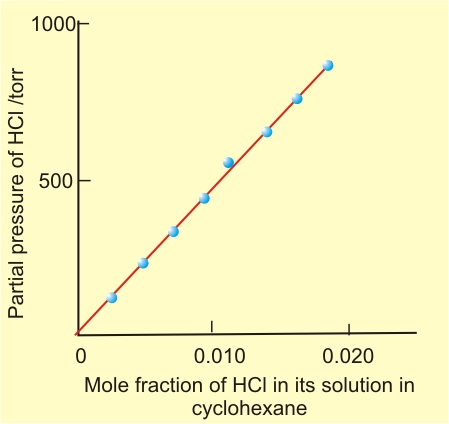
Here KH is the Henry’s law constant. If we draw a graph between partial pressure of the gas versus mole fraction of the gas in solution, then we should get a plot of the type as shown in figure given below.
Different gases have different KH values at the same temperature as shown in the given table. This suggests that KH is a function of the nature of the gas. It is obvious from the above equation that higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid. It can be seen from the given table that KH values for both N2 and O2 increase with increase of temperature indicating that the solubility of gases increases with decrease of temperature. It is due to this reason that aquatic species are more comfortable in cold waters rather than in warm waters.
| Gas | Temperature(K) | KH/kbar |
| Helium | 293 | 144.97 |
| Hydrogen | 293 | 69.16 |
| Nitrogen | 293 | 76.48 |
| Nitrogen | 303 | 88.84 |
| Oxygen | 293 | 34.86 |
| Oxygen | 303 | 46.82 |
| Argon | 298 | 40.3 |
| Carbon dioxide | 298 | 1.67 |
| Formaldehyde | 298 | 1.83x10-5 |
| Methane | 298 | 0.413 |
| Vinyl chloride | 298 | 0.611 |
There are various real-life examples of Henry's law. Some of them are mentioned below:
"Stay in the loop. Receive exam news, study resources, and expert advice!"
