Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record

Chemical Kinetics is one of the important chapters of class 12 Chemistry. It generally deals with understanding the rate of chemical reactions. Rate of chemical reaction is the speed at which chemical reaction takes place. Reaction rate is influenced by many factors including temperature, pressure, catalyst etc.
The National Testing Agency (NTA) has activated the NEET UG 2026 application form link on the official website. Students can register themselves at neet.nta.nic.in.
Reactions are classified in various orders like zero order reaction, First order reaction, second order reaction among others on the basis of rate law followed by them.
A first-order reaction refers to the reaction in which the rate of reaction is proportional to the first power of concentration of the reactant at any point of time. Hence, higher the concentration of reactant, higher will be the rate of reaction. Rate of first order reaction decreases as it proceeds due to decrease in concentration of reactant.
Hence in first order reaction, rate of reaction is directly proportional to the concentration of reactant as described in the equation below.
Reaction-
Reactant →Product
Rate =k[Reactant]
Below are some of the examples of First Order reaction
2N2O5 → 4NO2+O2
Rate = k[N2O5]
2H2O2 → 2H2O + O2
Rate = k[H2O2]
After analysing the last five years of NEET papers, it has been observed that, total 10 questions were asked from the Chemical Kinetics Chapter and out of those, four questions belong to First order reaction. This shows that First order reaction is the most important concept of Chemical Kinetics and most of the questions can be solved if Integral form of first order reaction is understood well. Questions asked from first order reactions are discussed in the article below.
Differential Rate Law for a First-Order Reaction reflects the change in concentration with respect to time which is shown by equation below:
Let us assume a first order reaction: A(Reactant)→B(Product)
Rate = -d[A]/dt = k[A]1 = k[A]
In the above equation,
‘k’ represents the rate constant of the first-order reaction, the unit of the rate constant is s-1.
‘[A]’ represents the concentration of the reactant.
d[A]/dt represents the change in the concentration of reactant ‘A’ with respect to time.
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Integrated Rate Law of first order reaction is used to calculate the value of rate of rate constant, concentration of reactant a given time, half life etc. Most of the numericals are solved by using Integrated rate law only. Differential rate law is used to obtain the Integral rate law of a First order reaction.
Differential rate law is: -d[A]/dt = k[A]
![]() d[A]/dt = -k[A]
d[A]/dt = -k[A]
Integrating both sides, we get
![]()
Where [A]o is concentration of reactant at time t0 s, and [A] is the concentration of reactant at time t. We get,
![]()
![]()
Considering t0 =0, and solving the equation, we get
![]() ln[A] – ln[A]0 = -kt
ln[A] – ln[A]0 = -kt
![]() ln[A] =ln[A]0 -kt
ln[A] =ln[A]0 -kt
Raising each side to component e, we get
![]()
![]()
![]()
![]()
Hence, ![]() is the final expression in the integrated form for first order reaction. The above derivation is useful in solving many numerical problems.
is the final expression in the integrated form for first order reaction. The above derivation is useful in solving many numerical problems.
Using the Integrated Rate law, Concentration v/s time Graph for First order reaction can be obtained which is shown below
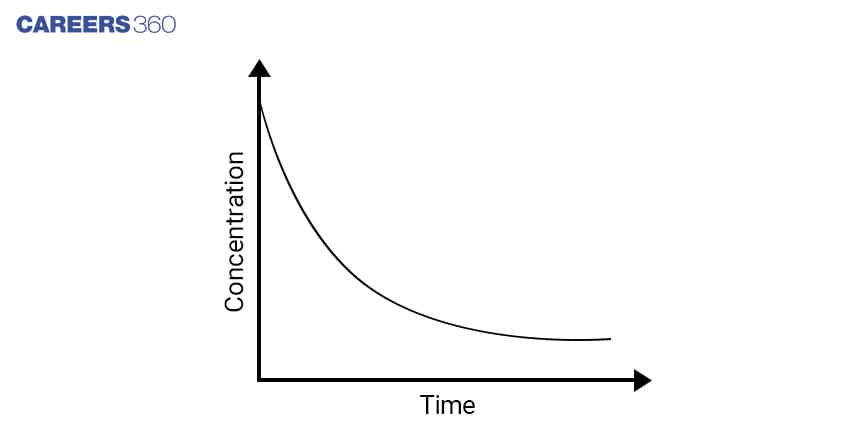
As the first-order reaction follows the equation ln[A] = ln[A]0 – kt which is similar to that of a straight line (y = mx + c) with slope -k. Hence, this equation can be plotted as-
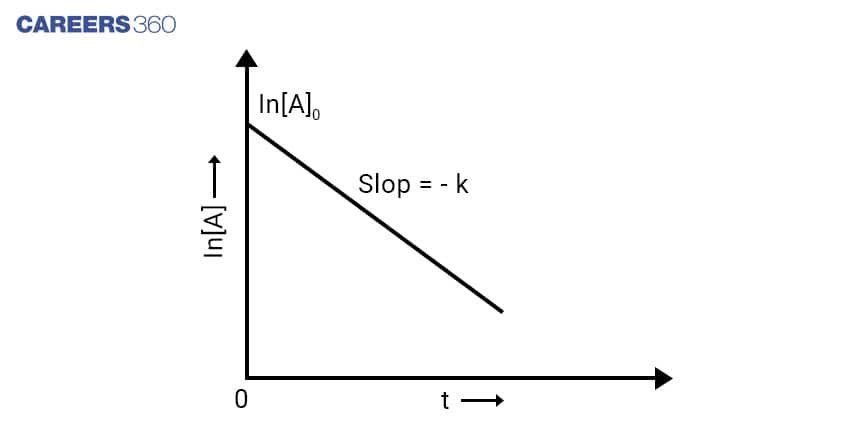
From the above graph, it can be said that the graph for ln[A] v/s t for a first-order reaction is a straight line with slope -k.
The half-life of a chemical reaction refers to the time required by the reactant concentration to become half of its initial value. It is represented by ![]() .
.
Hence, according to the first order reaction, ![]() . Where [A] refers to the concentration of the reactant at any time ‘‘t’’ and [A]o represents the initial concentration of the reactant.
. Where [A] refers to the concentration of the reactant at any time ‘‘t’’ and [A]o represents the initial concentration of the reactant.
At half life, t = t1/2 , [A] =A0/2
Putting the value of [A] =A0/2 and t = t1/2 in the equation ![]() , we get
, we get
![]()
![]()
Taking ln on both sides, we get,
![]()
![]()
Hence half life of the first order reaction is 0.693/k, where k is the rate constant.
Below discussed are some of the questions asked in the NEET exam from first order reaction.
Question 1. A first order reaction has a specific reaction rate of 10-2 sec-1. How much time will it take for 20 g of the reactant to reduce to 5 g? [NEET 2017]
238.6 sec
138.6 sec
346.5 sec
693.0 sec
Ans. The rate of first order reaction is proportional to the first power of the concentration of the reaction.
Let us consider a reaction, R → P , wherein
R → P
At t=0 a 0
At t=t a-x x
![]()
![]()
After solving this equation, we get
![]()
Now, putting the values
![]()
t= 138.6 sec
Alternatively, this can be solved by the half life method. According to which after the first half life concentration becomes half and after the second half life, concentration becomes ¼ th of the initial concentration. Hence after two half life, the concentration becomes from 20g to 5g.
![]()
![]()
![]()
Now, 2t1/2= 69.3×2= 138.6 sec.
Hence option ‘’C’’ is correct.
Question 2. The correct difference between first and second-order reactions is that [NEET 2018]
a first-order reaction can be catalysed; a second-order reaction cannot be catalysed
the half-life of a first-order reaction does not depend on [A]o; the half-life of a second-order reaction does depend on [A]o
the rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations
the rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations
Ans. For first Order reaction, rate is directly proportional to the first power of concentration.
For a reaction, A→B
Rate = -d[A]/dt = k[A] and half life is
![]()
Where unit of ![]() .
.
Hence ![]()
For second order reaction, For a reaction, A→B
Rate = ![]() and its half life is
and its half life is ![]()
![]()
Hence, The half-life of a first-order reaction does not depend on [A]o; the half-life of a second-order reaction does depend on [A]o
Option B is correct.
Question 3. If the rate constant for a first order reaction is k, the time (t) required for the completion of 99 per cent of the reaction is given by: [NEET 2019]
t = 0.693/k
t = 6.909/k
t = 4.606/k
t = 2.303/k
Ans. Let us consider a reaction, R → P , wherein
R → P
At t=0 a 0
At t=t a-x x
For the First Order reaction, Rate of reaction is proportional to the first power of the concentration of the reaction.
![]()
![]()
![]()
Unit of ![]()
![]() and
and
![]()
![]()
![]()
![]()
![]()
Hence option C is correct.
Question 4. The rate constant for a first order reaction is ![]() The time required to reduce 2 g of the reactant to 0.2g is: [NEET 2020]
The time required to reduce 2 g of the reactant to 0.2g is: [NEET 2020]
1000s
100s
200s
500s
Ans. Given,
![]()
Hence the given reaction is of first order.
Thus, we have:
![]()
Hence it is from the option of keeping th
![]()
Thus, ![]()
![]()
Therefore, Option(D) is correct.
On Question asked by student community
Hi Gawade,
please refer to this article -
https://medicine.careers360.com/articles/neet-ug-mock-tests
You can find the mock test link here
Government Medical Colleges in states like Rajasthan, Uttar Pradesh, Madhya Pradesh, Bihar, Haryana, Punjab, Gujarat, Maharashtra
Hi Arti,
Please refer to these links
Weightage:
https://medicine.careers360.com/articles/neet-biology-chapter-wise-weightage
High-weightage chapters:
https://medicine.careers360.com/articles/neet-2026-high-weightage-chapters
Do or Die Biology:
https://medicine.careers360.com/articles/do-or-die-chapters-in-biology-for-neet
Hindi syllabus:
https://medicine.careers360.com/hi/articles/neet-syllabus
Most Scoring Concepts eBook (free PDF):
https://medicine.careers360.com/download/ebooks/neet-most-scoring-chapters-topics-based-on-past-5-year-analysis
You still have enough time. For NEET 2026 (drop year, 3 months left), focus on:
NCERT linebyline for highweightage chapters (Human Physiology, Plant Physiology, Cell, Biomolecules, Biological Classification, Plant/Animal Kingdom, Genetics, Ecology)
Daily chapterwise MCQs + PYQs and weekly full mocks with proper analysis
Useful Careers360 links for planning:
NEET
The
NEET cut off 2025
for PwD (handicapped) candidates in Telangana was set at 40th percentile with 126 - 113 marks. Admission to government colleges in Telangana require higher marks as many as over 500 marks in NEET.
For more information, check the given below link.
https://medicine.careers360.com/articles/neet-cutoff-telangana
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Get Job-Ready with New-Age Allied Health Programmes
Get Job Ready in Healthcare | Employability-Focused Programs
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly