Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
The most frequently asked concepts in NEET Chemistry (2021–2025) reveal clear patterns in the type of questions repeatedly tested in the exam. Analysing these trends helps students understand which topics carry consistent weightage and require deeper preparation.
This Story also Contains
This guide provides a detailed overview of important NEET 2026 Chemistry topics, chapter-wise weightage from the last five years, and PYQ trends to help aspirants prepare strategically. By focusing on high-scoring and frequently repeated NEET exam concepts, students can optimise their preparation, improve accuracy, and avoid wasting time.
Let's understand the overall pattern of NEET Chemistry (2021–2025). The Chemistry section tests both conceptual clarity and application, with questions from physical, organic, and inorganic chemistry. Here’s a quick look at the question distribution, marks, and difficulty trends observed over the last five years:
Organic Chemistry questions have slightly increased since 2022, showing a stronger focus on reaction mechanisms and practical-based concepts.
Physical Chemistry remains calculation-based but often conceptual, testing the mole concepts, thermodynamics, and chemical kinetics.
Inorganic Chemistry questions are mostly fact-based and memory-driven, often repeated from previous years.
The overall difficulty level has remained moderate, with most questions derived directly from NCERT textbooks.
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Based on the analysis of NEET Chemistry papers from 2021 to 2025, certain chapters and concepts are repeatedly tested. Focusing on these high-frequency topics can help students maximise their scores. Here’s a list of the most important NEET 2026 chemistry concepts:
Basic Concepts of Chemistry: Stoichiometry, limiting reagent, and empirical formula questions appear every year.
Thermodynamics: Enthalpy, Hess’s law, and Gibbs' free energy are frequently tested.
Chemical Kinetics: Rate laws, order of reaction, and half-life calculations.
Equilibrium: Le Chatelier’s principle, equilibrium constants, ionic equilibria.
Alcohols, Phenols, and Ethers: Preparation, properties, and reactions.
Aldehydes, Ketones and Carboxylic Acids: Nucleophilic addition reactions, oxidation, reduction.
Amines: Basicity, preparation, reactions with nitrous acid.
Hydrocarbons: Alkanes, alkenes, alkynes - addition, substitution, combustion reactions.
Chemical Bonding and Molecular Structure: VSEPR, hybridisation, shapes, polarity.
Coordination Compounds: Ligands, bonding, nomenclature, and isomerism.
d and f Block Elements: Transition metals, complexes, colour, and magnetic properties.
Understanding the chapter-wise weightage helps students focus on topics that carry the most marks and appear frequently in NEET Chemistry from 2021 to 2025.
The data is from the past five years, and chapters States of Matter, Hydrogen, The s-Block Element, Solid State, Surface Chemistry, Polymers, Environmental Chemistry, General Principles and Processes of Isolation of Elements are not in the NEET 2026 syllabus now
|
Chapter |
2021 |
2022 |
2023 |
2024 |
2025 |
Total weightage (%) |
|
1 |
1 |
1 |
1 |
3 |
3.91 | |
|
3 |
3 |
2 |
3 |
2 |
5.12 | |
|
Classification of Elements & Periodicity |
0 |
0 |
4 |
3 |
4 |
5.57 |
|
4 |
3 |
3 |
3 |
2 |
6.12 | |
|
States of Matter |
3 |
3 |
2 |
0 |
0 |
3.34 |
|
3 |
2 |
1 |
4 |
3 |
5.15 | |
|
1 |
1 |
1 |
3 |
1 |
2.97 | |
|
1 |
1 |
1 |
1 |
1 |
2.82 | |
|
Hydrogen |
0 |
1 |
1 |
0 |
0 |
0.84 |
|
The s-Block Element |
2 |
1 |
1 |
0 |
0 |
1.25 |
|
3 |
4 |
2 |
2 |
0 |
3.58 | |
|
2 |
3 |
2 |
1 |
1 |
3.43 | |
|
2 |
1 |
2 |
6 |
5 |
6.94 | |
|
Solid State |
2 |
2 |
2 |
0 |
0 |
2.34 |
|
3 |
3 |
0 |
5 |
3 |
6.33 | |
|
1 |
2 |
2 |
1 |
0 |
2.32 | |
|
1 |
2 |
1 |
2 |
3 |
4.73 | |
|
Surface Chemistry |
0 |
0 |
2 |
0 |
0 |
0.87 |
|
0 |
2 |
3 |
4 |
4 |
5.44 | |
|
3 |
3 |
1 |
5 |
4 |
6.63 | |
|
3 |
2 |
1 |
2 |
2 |
4.28 | |
|
2 |
2 |
4 |
2 |
1 |
4.28 | |
|
5 |
2 |
3 |
0 |
1 |
4.7 | |
|
Amines |
2 |
2 |
4 |
0 |
3 |
3.66 |
|
1 |
0 |
1 |
2 |
2 |
2.6 | |
|
Polymers |
1 |
1 |
1 |
0 |
0 |
1.25 |
|
Environmental Chemistry |
0 |
1 |
1 |
0 |
0 |
0.87 |
|
General Principles and Processes of Isolation of Elements |
1 |
2 |
1 |
0 |
0 |
1.28 |
This section provides previous year NEET Chemistry questions (2021–2025) based on the most frequently asked concepts. Students can use these questions to practice high-yield topics, understand repeated patterns, and improve their accuracy in the Chemistry section.
Ques: For a reaction $A \rightarrow B$, the enthalpy of reaction is $\Delta H = -4.2\ \text{kJ mol}^{-1}$ and the enthalpy of activation is $E_a = 9.6\ \text{kJ mol}^{-1}$. The correct potential energy profile for the reaction is shown in the option. (NEET 2021)
Chapter: Chemical Kinetics
Option 1)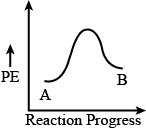
Option 2)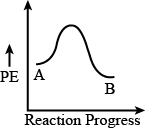
Option 3)
Option 4)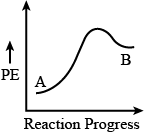
Correct answer: Option 2
Explanation: For an exothermic reaction, the enthalpy of the reactant will be greater than the enthalpy of the product and as a result, the energy profile diagram will look like
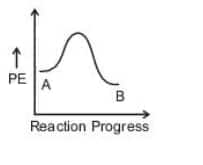
Hence, the answer is option (2).
Ques: Which one of the following alcohols reacts instantaneously with Lucas reagent? (NEET 2024)
Chapter: Alcohols Phenols and Ethers
Option 1) $CH_3 - CH_2 - CH_2 - CH_2OH$
Option 2)
Option 3)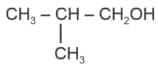
Option 4)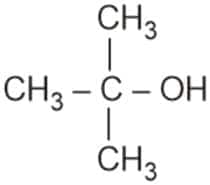
Correct answer: Option 4
Explanation: Tertiary alcohols react instantaneously with Lucas reagent and give immediate turbidity. In case of tertiary alcohols, they form halides easily with Lucas reagent.
Hence, the answer is option (4).
Ques: Given below are two statements:
Statement I: A hypothetical diatomic molecule with bond order zero is quite stable. Statement II: As the bond order increases, the bond length increases.
In light of the above statements, choose the most appropriate answer from the options given below:
Chapter: Chemical Bonding and Molecular Structure
Option 1) Statement 1 is false but Statement II is true
Option 2) Both Statement I and Statement II are true
Option 3) Both Statement I and Statement II are false
Option 4) Statement 1 is true but Statement II is false
Correct answer: Option 3
Explanation: A bond order of zero means no net bonding between the atoms, indicating the molecule cannot exist or is highly unstable. So the first statement is false
As the bond order increases, the bond length decreases because stronger bonding pulls the atoms closer together. And also the second statement is false
So the correct answer is (3) Both Statement I and Statement II are false
Hence, the correct answer is option (3).
Frequently Asked Questions (FAQs)
The basic concepts of chemistry for NEET include atomic structure, mole concept, chemical equations, stoichiometry, laws of chemical combination, concentration units, and dimensional analysis, which form the foundation for understanding physical, organic, and inorganic chemistry.
The hardest chapter in NEET Chemistry is often considered to be Thermodynamics or Organic Chemistry because they involve complex concepts and multiple formulas and require a deep understanding of theory as well as strong problem-solving skills.
The NEET 2025 Chemistry section was of moderate difficulty. Most questions were NCERT-based, but some required good conceptual understanding and application.
On Question asked by student community
Yes, it is an entrance exam. Please register at http://www.upvetuniv.edu.in/ .
Prospectus can be found at https://upvetuniv.edu.in/wp-content/uploads/2026/02/Prospectus-2026-27Final.pdf
You can check the NEET PYQs by Careers360 for previosu years' question papers.
Yes, You can change the date of birth in the NEET registration correction window.
To know what are the details can be corrected in the submitted NEET application form, Click here .
Yes — you can sit for NEET (National Eligibility cum Entrance Test) even if you took the Commerce stream in school, provided you meet the eligibility criteria set by the National Medical Commission (NMC) and your state/university. Key points and steps:
Eligibility criteria (core requirements)
Academic subjects: You must have
If your father’s name appears differently in your Class 10 marksheet compared to other documents, you should not upload an affidavit during the initial
NEET
application unless the form specifically asks for it.
Fill the application using the name that matches your Aadhaar and other main IDs, and prepare a
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly