Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
The Chemistry section has 45 questions out of the total of 180 questions in the NEET exam. The subject is further divided into Physical, Organic, and Inorganic Chemistry. Chemical Kinetics is a part of the NEET syllabus. Solving Chemical Kinetics NEET PYQ provides knowledge about the mechanisms and factors affecting reactions.
The National Testing Agency (NTA) has activated the NEET UG 2026 application form link on the official website. Students can register themselves at neet.nta.nic.in.
This Story also Contains

Clearing the NEET exam requires a smart and strategic approach as the syllabus is vast. Chemical Kinetics involves the theory concepts as well as the mathematical calculations. NEET PYQ chemical kinetics covers concepts like reaction order, half-life calculations, and real-world applications.
Chemical Kinetics helps aspirants learn about the Arrhenius equation and the collision theory of reactions. NEET chemistry chapter-wise weightage shows Chemical Kinetics holds 4.73% weightage. It is an important chapter of the NEET chemistry syllabus. Attempting NEET PYQ chemical kinetics helps students identify important topics. The table below shows the number of questions that appeared in the previous years.
Year | No. of Questions |
2025 | 3 |
2024 | 2 |
2023 | 1 |
2022 | 2 |
2021 | 1 |
Numerical-based questions from Chemical Kinetics appear in the NEET exam. To solve them in less time, an effective NEET preparation timetable is important. The timetable should include 2-3 hours daily for solving the NEET question paper. Provided below are a few NEET chemical kinetics previous year questions with solutions. Going through them makes students familiar with the question pattern.
Question 1: If the half-life
Options
10 minutes
2 minutes
4 minutes
5 minutes
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Answer: For a first-order reaction-
Also half-life for a first-order is given by
Hence, the correct answer is option 1) 10 minutes.
Question 2: If the rate constant of a reaction is
Options
21.0 s
69.3 s
23.1 s
210 s
Answer: Given that
Initial concentration
Final concentration
Rate constant
Given
Since the units of
Formula:
Substitute:
This becomes
Now,
So,
Hence, the correct answer is option 2) 69.3 s.
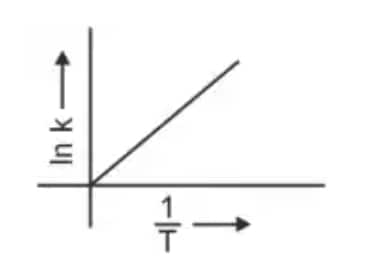
Question 3: Which plot of In k vs 1/T is consistent with the Arrhenius equation? (NEET 2024)
Options
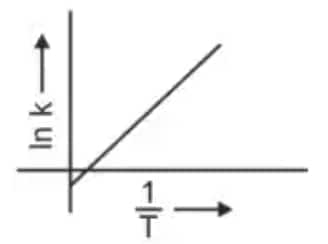
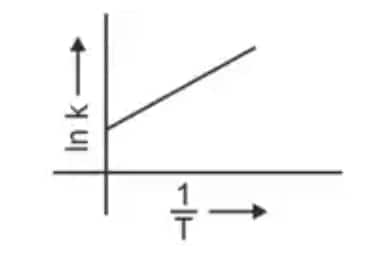
3. 
4. 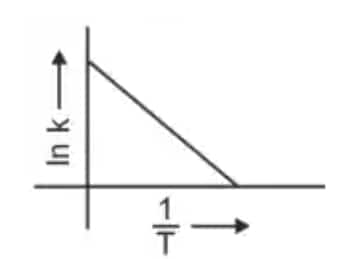
Answer: The Arrhenius equation is given as
In

Hence, the correct answer is option (4) 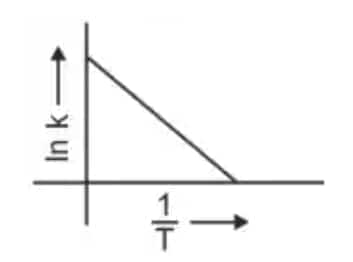
Question 4: For a certain reaction, the rate
Options
decrease by a factor of nine.
increase by a factor of six.
increase by a factor of nine
increase by a factor of three
Answer:
So, the rate will increase 9 times the initial rate.
Hence, the correct answer is option (3), increase by a factor of nine.
Question 5: Given below are two statements: one is labeled as Assertion A and the other is labeled as Reason R :
Assertion A: A reaction can have zero activation energy.
Reasons R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to the threshold value is called activation energy.
In light of the above statements, choose the correct answer from the options given below: (NEET 2023)
Options
Both A and R are true, and R is the correct explanation of A.
Both A and R are true, and R is NOT the correct explanation of A.
A is true, but R is false.
A is false, but R is true.
Answer: The free radical reactions can have zero activation energy. In the free radical combination reaction in termination, steps have
Hence, the correct answer is option (2), Both A and R are true, and R is NOT the correct explanation of A.
Question 6: For a first-order reaction
Options
0.9212
0.4606
0.2303
1.3828
Answer: For a first-order reaction, the integrated rate law expression is
Hence, the correct answer is option (1), 0.9212.
Question 7: The given graph is a representation of the kinetics of a reaction
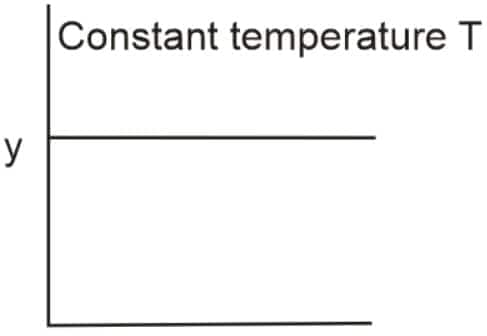
The y and x axes for zero and first-order reactions, respectively, are: (NEET 2022)
Options
zero order (y= concentration and x= time), first order (y= rate constant and x= concentration)
zero order (
zero order (
zero order (
Answer: For a zero-order reaction:
Rate
For a first-order reaction:
Hence, the correct answer is option (2), zero order (
Question 8: The slope of the Arrhenius Plot
[Given
Options:
1. \( 41.5 \ \text{kJ mol}^{-1} \)
2. \( 83.0 \ \text{kJ mol}^{-1} \)
3. \( 166 \ \text{kJ mol}^{-1} \)
4. \( -83 \ \text{kJ mol}^{-1} \)
Answer: According to the Arrhenius equation
\( k = Ae^{-E_a/RT} \)
\( \ln k = \ln A + \ln e^{-E_a/RT} \)
\( \ln k = \ln A - \frac{E_a}{R} \left( \frac{1}{T} \right) \)
The slope \( \left( \ln k \ \text{vs} \ \frac{1}{T} \right) \) of the above equation
\( m = -\frac{E_a}{R} \)
Given, \( m = -5 \times 10^3 \ \text{K} \) and \( R = 8.314 \ \text{J K}^{-1} \text{mol}^{-1} \)
\( -5 \times 10^3 = -\frac{E_a}{8.314} \)
\( E_a = 5 \times 10^3 \times 8.314 \ \text{J/mol} \)
\( E_a = 41.57 \times 10^3 \ \text{J/mol} \)
\( E_a = 41.5 \ \mathrm{kJ \ mol^{-1}} \)
Hence, the correct answer is option (1), \( 41.5 \ \text{kJ mol}^{-1} \).
Question 9: An increase in the concentration of the reactants of a reaction leads to a change in: (NEET 2020)
Options
Collision frequency
Activation energy
heat of reaction
threshold energy
Answer: An increase in the concentration of the reactants leads to an increase in the collision frequency. Threshold energy, activation energy, and heat of reaction are not affected by the increase in concentration. This is because, due to the increase in concentration, the number of particles increases and hence the number of collisions.
Hence, the correct answer is option (1), Collision frequency.
Question 10: The rate constant for a first-order reaction is
Options
1000s
100s
200s
500s
Answer: We are given:
Thus, the reaction is of the first order.
We have:
Thus,
Hence, the correct answer is option (4), 500s.
Chemical Kinetics is a scoring unit in chemistry. It is among the do-or-die chapters for NEET. Questions in the NEET 2024 question paper come from the Chemical Kinetics unit. Chemical kinetics NEET PYQ includes questions on how reactions occur and the different factors affecting them. Students thinking about how to study chemistry for NEET should start with this unit. Given below are a few important topics from this unit.
Topics | Description |
Defined as the change in concentration of reactants or products per unit time. | |
Order of Reaction | For complex reactions, the order is determined by the rate-determining step. |
The number of reacting species involved in an elementary reaction. | |
Rate Constant (k) | A proportionality constant that relates the rate of the reaction to the concentrations of the reactants. |
Activation Energy (Eₐ) | The minimum energy required for a reaction to occur. |
This equation relates the rate constant to temperature and activation energy. It is important for understanding how temperature affects reaction rates. | |
Half-Life (t₁/₂) | The time required for half of the reactant to be consumed is known as half half-life. |
Factors Affecting Reaction Rates | Understanding how concentration, temperature, and catalysts influence reaction rates is important. |
Solving previous year questions regularly builds confidence. It helps students understand the types of questions asked in the exam. Aspirants can start by solving the 20 important NEET chemistry questions to make their preparation strong. Given below are a few tips that they can use to solve NEET chemical kinetics previous year questions.
Students have to understand how to obtain rate laws from the rate-determining step. Accessing the NEET chemistry mock test improves score and speed.
Learn how to determine the order of a reaction from experimental data and not just by stoichiometry.
Get familiar with the rate equations for zero, first, and second-order reactions. Study only from the Chemistry books for NEET to avoid confusion.
Understand the role of activation energy and how it plays a part in the rate determination. Try to apply the Arrhenius equation to the temperature changes.
Solve NEET PYQ chemical kinetics by using the NEET chemistry question paper.
Prepare flashcards of important formulas for NEET chemistry. This helps to answer NEET chemical kinetics previous year questions confidently.
On Question asked by student community
Hi Gawade,
please refer to this article -
https://medicine.careers360.com/articles/neet-ug-mock-tests
You can find the mock test link here
Government Medical Colleges in states like Rajasthan, Uttar Pradesh, Madhya Pradesh, Bihar, Haryana, Punjab, Gujarat, Maharashtra
Hi Arti,
Please refer to these links
Weightage:
https://medicine.careers360.com/articles/neet-biology-chapter-wise-weightage
High-weightage chapters:
https://medicine.careers360.com/articles/neet-2026-high-weightage-chapters
Do or Die Biology:
https://medicine.careers360.com/articles/do-or-die-chapters-in-biology-for-neet
Hindi syllabus:
https://medicine.careers360.com/hi/articles/neet-syllabus
Most Scoring Concepts eBook (free PDF):
https://medicine.careers360.com/download/ebooks/neet-most-scoring-chapters-topics-based-on-past-5-year-analysis
You still have enough time. For NEET 2026 (drop year, 3 months left), focus on:
NCERT linebyline for highweightage chapters (Human Physiology, Plant Physiology, Cell, Biomolecules, Biological Classification, Plant/Animal Kingdom, Genetics, Ecology)
Daily chapterwise MCQs + PYQs and weekly full mocks with proper analysis
Useful Careers360 links for planning:
NEET
The
NEET cut off 2025
for PwD (handicapped) candidates in Telangana was set at 40th percentile with 126 - 113 marks. Admission to government colleges in Telangana require higher marks as many as over 500 marks in NEET.
For more information, check the given below link.
https://medicine.careers360.com/articles/neet-cutoff-telangana
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Get Job-Ready with New-Age Allied Health Programmes
Get Job Ready in Healthcare | Employability-Focused Programs
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly