JSS University Mysore 2025
NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs
Thermodynamics physics NEET questions: With a 25% weightage for the Chemistry section, the National Entrance Test for Medical Colleges in India, NEET covers 19 units, from basic concepts to electrochemistry and organic and inorganic chemistry. A strong knowledge of chemistry secures good scores and also provides the groundwork for a career in medicine and research.
This Story also Contains

Of the 19 units in the chemistry section of the NEET exam syllabus, Thermodynamics usually account for 8% of the total exam score weightage. The most repeated core concepts in the Thermodynamics article are designed to help you focus your revision on the thermodynamics NEET previous year question paper. These thermodynamics NEET pyq have appeared in the past 10 years of NEET question papers.
Given Below is the detailed analysis of Thermodynamics physics NEET questions
Marks Contribution: Approximately 4-8 marks in the NEET Chemistry paper.
Number of Questions: Around 1-2 questions per exam, with some questions integrating numerical-based or process-oriented characteristics.
Every year, 1-2 questions are consistently asked from this chapter in the last 10 years NEET question paper thermodynamics physics NEET questions.
Subtopic | Approximate Weightage | Key Focus Areas | ||
Basic Concepts of Thermodynamics | 10-12% | Definitions, Identifying system types, Difference between state and path functions | ||
Laws of Thermodynamics | 15-18% | Application of first law, Concept of entropy, Spontaneity of reactions | ||
Enthalpy & Internal Energy | 10-12% | Calculation of heat and work, Expansion work (PV work), Endothermic vs. Exothermic reactions | ||
Entropy & Gibbs Free Energy | 12-15% | ΔG = ΔH - TΔS, Conditions for spontaneity, Predicting feasibility of reactions | ||
Hess's Law & Bond Enthalpy | 8-10% | Using Hess’s Law for reaction enthalpy, calculating enthalpy changes using bond enthalpies | ||
Thermodynamic Equilibrium & Reversibility | 5-8% | Conditions for reversible reactions, Work done in reversible vs. irreversible processes | ||
Relation with Chemical Equilibrium | 8-10% | Relationship between ΔG and K, Effect of temperature on equilibrium | ||
Biological Relevance of Thermodynamics | 5-7% | Role of ATP in energy transfer, Thermodynamics in biological reactions |
Also Read
To do well in thermodynamics physics NEET questions, students must thoroughly understand key concepts like the laws of thermodynamics, thermodynamic processes, and thermodynamic properties such as internal energy, enthalpy, and entropy.
thermodynamics NEET questions often involve calculating work done, heat transfer, and changes in thermodynamic properties during various processes, including isothermal, adiabatic, isobaric, and isochoric processes.
Practising thermodynamics physics NEET questions is important for learning, as it helps students become familiar with the exam pattern and improve their problem-solving skills.
Understanding the first, second, and zeroth laws of thermodynamics is important for solving thermodynamics NEET pyq, as these laws form the foundation of thermodynamic principles.
Thermodynamics NEET questions often require the integration of concepts from both chemistry and physics, such as enthalpy changes in chemical reactions and the behaviour of gases in different processes.
NEET thermodynamics questions typically include multiple-choice questions (MCQs) that test students' ability to apply thermodynamic principles to solve problems and understand complex processes.
Some common mistakes students make while attempting NEET thermodynamics questions:
Confusion Between Enthalpy and Entropy: Many students struggle to differentiate between enthalpy (ΔH) and entropy (ΔS). Enthalpy refers to heat content, while entropy measures disorder in a system. Misunderstanding these terms can lead to errors in predicting spontaneity (ΔG = ΔH - TΔS) of reactions.
Incorrect Application of Gibbs Free Energy: A common mistake is using Gibbs free energy (ΔG) incorrectly when determining reaction feasibility. Students often miscalculate temperature dependence in ΔG = ΔH - TΔS, forgetting that T must be in Kelvin (K) and not Celsius.
Sign Errors in First Law of Thermodynamics: Students frequently confuse the sign conventions in ΔU = Q + W. They forget that work done on the system is positive, while work done by the system is negative. Such errors can lead to incorrect energy calculations in numerical problems.
Misunderstanding Heat Capacity (Cp vs. Cv): Confusing Cp (at constant pressure) and Cv (at constant volume) is a common issue. Many students forget the relation Cp - Cv = R and struggle with molar heat capacity calculations in different conditions, leading to mistakes in solving thermodynamic problems.
Here are some questions from Thermodynamics NEET PYQs:
Q1. Which of the following options is the correct relation between change in enthalpy and change in internal energy?
NEET 2023
Difficulty level: Easy
Chapter: Thermodynamics
Option 1: ![]()
Option 2: ![]()
Option 3: ![]()
Option 4: ![]()
Solution:
Relation between ![]()
![]()
Hence, the answer is option (2).
Q2. Which of the following p.V curves represents the maximum work done?
NEET 2022
Difficulty level: Medium
Chapter: Thermodynamics
Option 1: 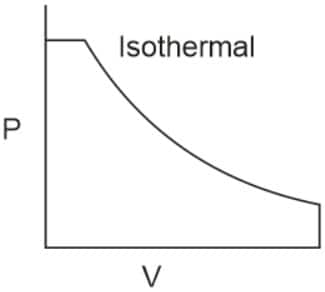
Option 2: 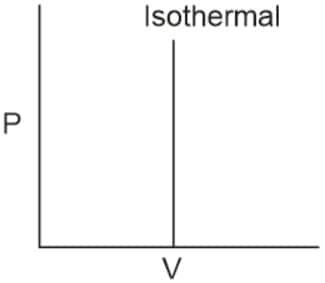
Option 3: 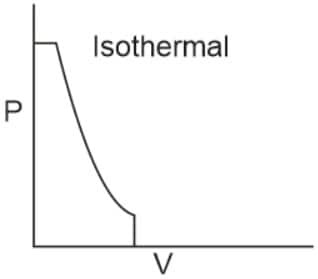
Option 4: 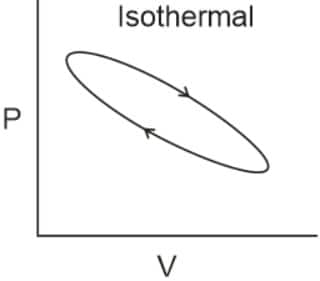
Solution:
In the P_V curve, the work is equal to the area under the P_V curve.
Graph (I) has the maximum area under the curve, so it will have a maximum area under the curve.
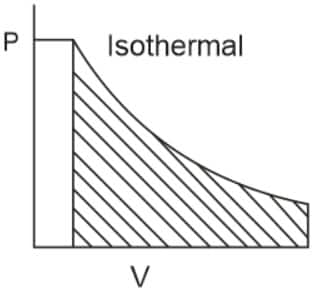
Hence, the answer is option (1).
Q3. The correct option for free expansion of an ideal gas under adiabatic conditions is:
NEET 2020
Difficulty level: Difficult
Chapter: Thermodynamics
Option 1: ![]()
Option 2:![]()
Option 3: ![]()
Option 4: ![]()
Solution:
For an adiabatic process, ![]() and free expansion,
and free expansion, ![]() , Therefore
, Therefore ![]() .
.
The temperature during adiabatic expansion does not change.
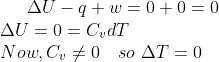
Hence, the answer is option (2).
Q4. For the irreversible expansion of an ideal gas under isothermal conditions, the correct option is :
NEET 2021
Difficulty level: Difficult
Chapter: Thermodynamics
Option 1: ![]()
Option 2: ![]()
Option 3: ![]()
Option 4: ![]()
Solution:
For the irreversible expansion of an ideal gas under isothermal conditions -
We know that for a spontaneous process, ![]() and since the irreversible process is always spontaneous, therefore
and since the irreversible process is always spontaneous, therefore ![]() .
.
![]() for the isothermal process.
for the isothermal process.
Now, ![]()
So, ![]()
Correct option ![]() .
.
Hence, the answer is option (3).
Q5. Which of the following is the correct option for the right relationship between ![]() and
and ![]() for one mole of ideal gas?
for one mole of ideal gas?
NEET 2021
Difficulty level: Difficult
Chapter: Thermodynamics
Option 1: ![]()
Option 2: ![]()
Option 3: ![]()
Option 4: ![]()
Solution:
The relation between ![]() for one mole of an ideal gas is given as
for one mole of an ideal gas is given as
![]() .
.
Hence, the answer is option (3).
Increased practice from thermodynamics neet previous year question paper improves confidence in solving thermodynamics questions.
Knowledge about the pattern of the exam reduces stress during the exam.
Enhances the efficiency in solving questions within the time limit.
Also Read:
On Question asked by student community
Hello,
Here are good career options for PCB students in India (excluding NEET/MBBS) that have high demand, jobs available, and long-term stability :
1. Biotechnology
Study: B.Sc/ B.Tech/ M.Sc in Biotechnology.
Jobs: Research, labs, pharma, agriculture biotech.
Why: Growing field with many industries.
2. Pharmacy
Study: B.Pharm, M.Pharm.
Jobs: Pharmacist,
Hi dear candidate,
You can prepare yourself for the NEET exam in the time span of 2 months with good amount of dedication and ample of practice. You can attempt the mock tests on our official website for free, practice PYQs and get to know the strategy tips as well.
HELLO,
Yes , the NEST exam is generally considered tougher than NEET as it requires more focus towards conceptual understanding and thinking , while NEET generally tests NCERT based knowledge where as in NEST it requires you to have deeper clarity in Physics , Chemistry , Biology and Mathematics.
NEET
Hello,
The National Eligibility cum Entrance Test (NEET) is conducted by the National Testing Agency (NTA) for admission to medical and dental colleges in India. Biology is the most important section in NEET as it carries 360 marks, which is half of the total score, with 45 questions each from
HELLO,
Below i am attaching the link through which you can easily access the previous three year question paper of NEET with solutions PDF
Here is the link :- https://medicine.careers360.com/download/sample-papers/neet-previous-year-question-papers-solutions-pdf
Hope this will help you!
NAAC A+ Accredited| Ranked #24 in University Category by NIRF | Applications open for multiple UG & PG Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available