Virohan Allied & Healthcare Programs
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Chemistry is one of the most scoring sections in NEET and can significantly improve the overall rank. With 45 questions in the exam, focusing on high-weightage topics is key to increasing the score. Many NEET aspirants search for important chemistry questions for NEET and practice material to revise quickly and improve accuracy.
This Story also Contains
.jpg)
To make preparation easier, the top 20 most important NEET Chemistry questions are provided. These questions cover Physical, Organic, and Inorganic Chemistry from high-weightage chapters, based on previous year trends. A free NEET Chemistry PDF with answers is also available, making it easy to revise anytime and strengthen your preparation for NEET exam.
The NEET 2026 chemistry has all three parts, which are Physical, Organic, and Inorganic Chemistry from Class 11 and Class 12. The table below shows the last 5 years weighatge analysis of NEET chemistry important chapters. Aspirants will also get to know the difficulty level of NEET chemistry important questions.
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
The data is from the last five years and chapters States of Matter, Hydrogen, The s-Block Element, Solid State, Surface Chemistry, Polymers, Environmental Chemistry, General Principles and Processes of Isolation of Elements are not in the NEET syllabus now
| Chapter | 2021 | 2022 | 2023 | 2024 | 2025 |
Total weightage (%) |
| Basic Concepts of Chemistry | 1 | 1 | 1 | 1 | 3 | 3.91 |
| Structure of Atom | 3 | 3 | 2 | 3 | 2 | 5.12 |
| Classification of Elements & Periodicity | 0 | 0 | 4 | 3 | 4 | 5.57 |
| Chemical Bonding and Molecular Structure | 4 | 3 | 3 | 3 | 2 | 6.12 |
| States of Matter | 3 | 3 | 2 | 0 | 0 | 3.34 |
| Thermodynamics | 3 | 2 | 1 | 4 | 3 | 5.15 |
| Equilibrium | 1 | 1 | 1 | 3 | 1 | 2.97 |
| Redox Reactions | 1 | 1 | 1 | 1 | 1 | 2.82 |
| Hydrogen | 0 | 1 | 1 | 0 | 0 | 0.84 |
| The s-Block Element | 2 | 1 | 1 | 0 | 0 | 1.25 |
| Some p-Block Elements | 3 | 4 | 2 | 2 | 0 | 3.58 |
| d and f Block Elements | 2 | 3 | 2 | 1 | 1 | 3.43 |
| Coordination Compounds | 2 | 1 | 2 | 6 | 5 | 6.94 |
| Solid State | 2 | 2 | 2 | 0 | 0 | 2.34 |
| Solutions | 3 | 3 | 0 | 5 | 3 | 6.33 |
| Electrochemistry | 1 | 2 | 2 | 1 | 0 | 2.32 |
| Chemical Kinetics | 1 | 2 | 1 | 2 | 3 | 4.73 |
| Surface Chemistry | 0 | 0 | 2 | 0 | 0 | 0.87 |
| Organic Chemistry – Some Basic Principles and Techniques | 0 | 2 | 3 | 4 | 4 | 5.44 |
| Hydrocarbons | 3 | 3 | 1 | 5 | 4 | 6.63 |
| Haloalkanes and Haloarenes | 3 | 2 | 1 | 2 | 2 | 4.28 |
| Alcohols, Phenols and Ethers | 2 | 2 | 4 | 2 | 1 | 4.28 |
| Aldehydes, Ketones and Carboxylic Acids | 5 | 2 | 3 | 0 | 1 | 4.7 |
| Amines | 2 | 2 | 4 | 0 | 3 | 3.66 |
| Biomolecules | 1 | 0 | 1 | 2 | 2 | 2.6 |
| Polymers | 1 | 1 | 1 | 0 | 0 | 1.25 |
| Environmental Chemistry | 0 | 1 | 1 | 0 | 0 | 0.87 |
| General Principles and Processes of Isolation of Elements | 1 | 2 | 1 | 0 | 0 | 1.28 |
This part is a combination of concept, numerical, and application-type questions of Physical, Organic, and Inorganic Chemistry. These are the most commonly asked questions. This will not only improve your NEET preparation but also improve speed and accuracy during the exam. We have presented here the most relevant NEET Chemistry questions along with detailed answers so that you can approach the exam questions confidently and effectively.
Ques:
Given below are two statements :
Statement I : A hypothetical diatomic molecule with bond order zero is quite stable. Statement II : As bond order increases, the bond length increases.
In the light of the above statements, choose the most appropriate answer from the options given below :
Option 1) Statement 1 is false but Statement II is true
Option 2) Both Statement I and Statement II are true
Option 3) Both Statement I and Statement II are false
Option 4) Statement 1 is true but Statement II is false
Chapter - Chemical bonding and molecular structure
Difficulty level - Easy
Solution -
A bond order of zero means no net bonding between the atoms, indicating the molecule cannot exist or is highly unstable. so the first statement is false
As bond order increases, bond length decreases because stronger bonding pulls the atoms closer together. and also the second statement is false
So the correct answer is (3) Both Statement I and Statement II are false
Hence, the correct answer is option (3).
Ques:
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : 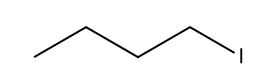
undergoes SN2 reaction faster than
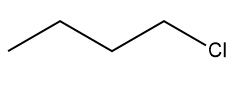
Reason ( RR ) : lodine is a better leaving group because of its large size.
In the light of the above statements, choose the correct answer from the options given below :
Option 1) A is false but R is true
Option 2) Both A and R are true and R is the correct explanation of A
Option 3) Both A and R are true but R is not the correct explanation of A
Option 4) A is true but R is false
Chapter - Haloalkanes and Haloarenes
Difficulty level - Medium
Solution -
Rate α [RX][Z-]
where Z- is the leaving group
Since iodide is a better leaving group than chloride so the SN2 reaction will be faster in the iodobutane.
The larger size of iodine results in a weaker bond between iodine and the carbon atom it's attached to that makes it easier to dissociate and leave during a reaction.
Hence, the correct answer is option (2).
Ques:
C( s)+2H2( g)→CH4( g);ΔH=−74.8 kJ mol−1
Which of the following diagrams gives an accurate representation of the above reaction? [R→ reactants; P→ products ]
Option 1) 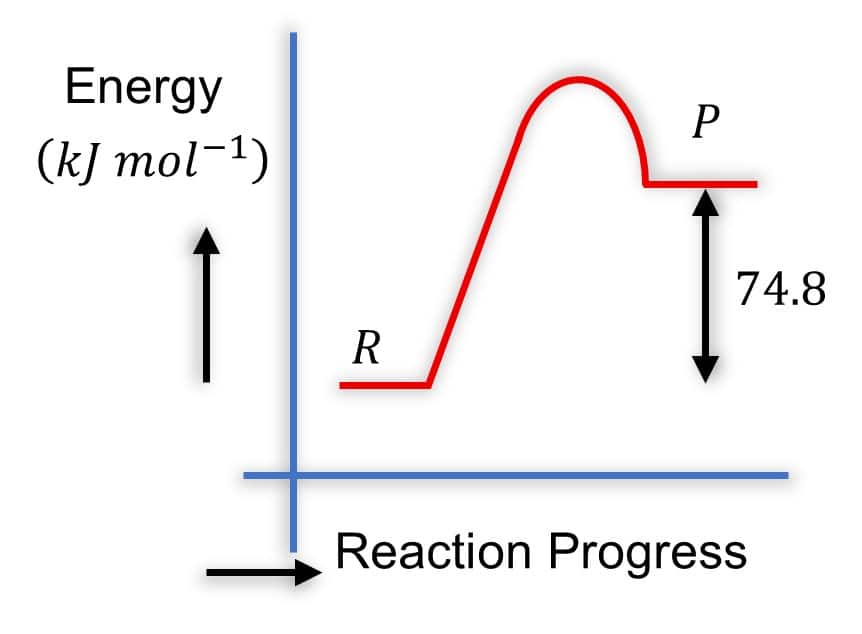
Option 2) 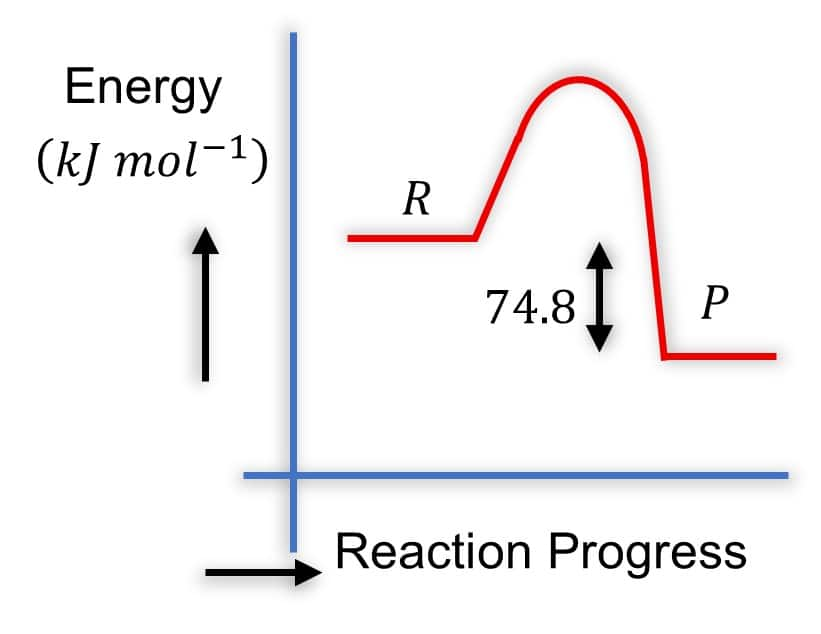
Option 3) 
Option 4) 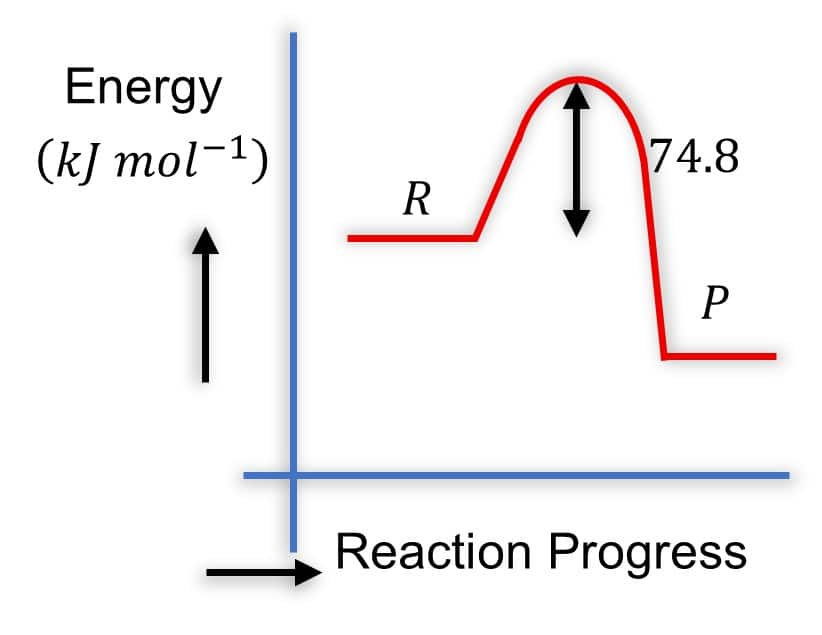
Chapter - Thermodynamics
Difficulty Level - Medium
Solution - Diagram 2:
- R is higher than P
- P is lower in energy
- The arrow from P to R is labeled 74.8 and points upward
- This is correct because it represents the energy difference, and ΔH=−74.8kJmol−1.
The arrow shows that products are lower by 74.8kJmol−1.
- It is common to show the magnitude of ΔH with an upward arrow between P and R even in exothermic reactions, as long as positions are correct.
Hence, the correct answer is option (2).
|
NEET Chemistry Important Questions |
To perform well in NEET Chemistry and score well, use these tried and tested techniques:
On Question asked by student community
Hi Gawade,
please refer to this article -
https://medicine.careers360.com/articles/neet-ug-mock-tests
You can find the mock test link here
Government Medical Colleges in states like Rajasthan, Uttar Pradesh, Madhya Pradesh, Bihar, Haryana, Punjab, Gujarat, Maharashtra
Hi Arti,
Please refer to these links
Weightage:
https://medicine.careers360.com/articles/neet-biology-chapter-wise-weightage
High-weightage chapters:
https://medicine.careers360.com/articles/neet-2026-high-weightage-chapters
Do or Die Biology:
https://medicine.careers360.com/articles/do-or-die-chapters-in-biology-for-neet
Hindi syllabus:
https://medicine.careers360.com/hi/articles/neet-syllabus
Most Scoring Concepts eBook (free PDF):
https://medicine.careers360.com/download/ebooks/neet-most-scoring-chapters-topics-based-on-past-5-year-analysis
You still have enough time. For NEET 2026 (drop year, 3 months left), focus on:
NCERT linebyline for highweightage chapters (Human Physiology, Plant Physiology, Cell, Biomolecules, Biological Classification, Plant/Animal Kingdom, Genetics, Ecology)
Daily chapterwise MCQs + PYQs and weekly full mocks with proper analysis
Useful Careers360 links for planning:
NEET
The
NEET cut off 2025
for PwD (handicapped) candidates in Telangana was set at 40th percentile with 126 - 113 marks. Admission to government colleges in Telangana require higher marks as many as over 500 marks in NEET.
For more information, check the given below link.
https://medicine.careers360.com/articles/neet-cutoff-telangana
Allied & Healthcare programs | 20+ Partner Universities & Institutes | 98% placement record
Get Job-Ready with New-Age Allied Health Programmes
Get Job Ready in Healthcare | Employability-Focused Programs
Amongst top 3% universities globally (QS Rankings) | Wide Range of scholarships available
Ranked #19 by NIRF, NAAC A++ Accredited | Recognized by dental council of India
Ranked #18 by NIRF, NAAC A++ Accredited | Unmatched clinical exposure with over 7 lakh patients yearly